You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 Atomic structure and the periodic table<br />
Reactions of elements<br />
KEY TERM<br />
Element A substance containing only<br />
one type of atom. A substance that<br />
cannot be broken down into simpler<br />
substances by chemical methods.<br />
TIP<br />
When writing symbols you must be<br />
very careful to ensure your capital<br />
letters cannot be mistaken for small<br />
letters and vice versa.<br />
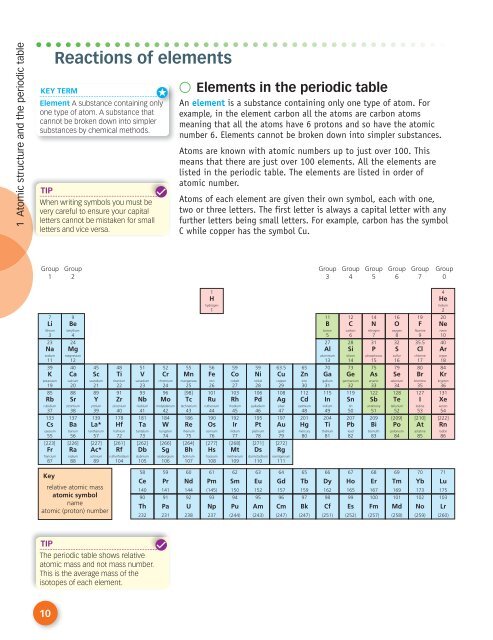
● Elements in the periodic table<br />
An element is a substance containing only one type of atom. For<br />
example, in the element carbon all the atoms are carbon atoms<br />
meaning that all the atoms have 6 protons and so have the atomic<br />
number 6. Elements cannot be broken down into simpler substances.<br />
Atoms are known with atomic numbers up to just over 100. This<br />
means that there are just over 100 elements. All the elements are<br />
listed in the periodic table. The elements are listed in order of<br />
atomic number.<br />
Atoms of each element are given their own symbol, each with one,<br />
two or three letters. The first letter is always a capital letter with any<br />
further letters being small letters. For example, carbon has the symbol<br />
C while copper has the symbol Cu.<br />
Group<br />
1<br />
Group<br />
2<br />
Group<br />
3<br />
Group<br />
4<br />
Group<br />
5<br />
Group<br />
6<br />
Group<br />
7<br />
Group<br />
0<br />
7<br />
Li<br />
lithium<br />
3<br />
23<br />
Na<br />
sodium<br />
11<br />
39<br />
K<br />
potassium<br />
19<br />
85<br />
Rb<br />
rubidium<br />
37<br />
133<br />
Cs<br />
caesium<br />
55<br />
[223]<br />
Fr<br />
francium<br />
87<br />
9<br />
Be<br />
beryllium<br />
4<br />
24<br />
Mg<br />
magnesium<br />
12<br />
40<br />
Ca<br />
calcium<br />
20<br />
88<br />
Sr<br />
strontium<br />
38<br />
137<br />
Ba<br />
barium<br />
56<br />
[226]<br />
Ra<br />
radium<br />
88<br />
45<br />
Sc<br />
scandium<br />
21<br />
89<br />
Y<br />
yttrium<br />
39<br />
139<br />
La*<br />
lanthanum<br />
57<br />
[227]<br />
Ac*<br />
actinium<br />
89<br />
48<br />
Ti<br />
titanium<br />
22<br />
91<br />
Zr<br />
zirconium<br />
40<br />
178<br />
Hf<br />
hafnium<br />
72<br />
[261]<br />
Rf<br />
rutherfordium<br />
104<br />
51<br />
V<br />
vanadium<br />
23<br />
93<br />
Nb<br />
niobium<br />
41<br />
181<br />
Ta<br />
tantalum<br />
73<br />
[262]<br />
Db<br />
dubnium<br />
105<br />
52<br />
Cr<br />
chromium<br />
24<br />
96<br />
Mo<br />
molybdenum<br />
42<br />
184<br />
W<br />
tungsten<br />
74<br />
[266]<br />
Sg<br />
seaborgium<br />
106<br />
55<br />
Mn<br />
manganese<br />
25<br />
[98]<br />
Tc<br />
technetium<br />
43<br />
186<br />
Re<br />
rhenium<br />
75<br />
[264]<br />
Bh<br />
bohrium<br />
107<br />
1<br />
H<br />
hydrogen<br />
1<br />
56<br />
Fe<br />
iron<br />
26<br />
101<br />
Ru<br />
ruthenium<br />
44<br />
190<br />
Os<br />
osmium<br />
76<br />
[277]<br />
Hs<br />
hassium<br />
108<br />
59<br />
Co<br />
cobalt<br />
27<br />
103<br />
Rh<br />
rhodium<br />
45<br />
192<br />
Ir<br />
iridium<br />
77<br />
[268]<br />
Mt<br />
meitnerium<br />
109<br />
59<br />
Ni<br />
nickel<br />
28<br />
106<br />
Pd<br />
palladium<br />
46<br />
195<br />
Pt<br />
platinum<br />
78<br />
[271]<br />
Ds<br />
darmstadtium<br />
110<br />
63.5<br />
Cu<br />
copper<br />
29<br />
108<br />
Ag<br />
silver<br />
47<br />
197<br />
Au<br />
gold<br />
79<br />
[272]<br />
Rg<br />
roentgenium<br />
111<br />
65<br />
Zn<br />
zinc<br />
30<br />
112<br />
Cd<br />
cadmium<br />
48<br />
201<br />
Hg<br />
mercury<br />
80<br />
11<br />
B<br />
boron<br />
5<br />
27<br />
Al<br />
aluminium<br />
13<br />
70<br />
Ga<br />
gallium<br />
31<br />
115<br />
In<br />
indium<br />
49<br />
204<br />
Ti<br />
thallium<br />
81<br />
12<br />
C<br />
carbon<br />
6<br />
28<br />
Si<br />
silicon<br />
14<br />
73<br />
Ge<br />
germanium<br />
32<br />
119<br />
Sn<br />
tin<br />
50<br />
207<br />
Pb<br />
lead<br />
82<br />
14<br />
N<br />
nitrogen<br />
7<br />
31<br />
P<br />
phosphorus<br />
15<br />
75<br />
As<br />
arsenic<br />
33<br />
122<br />
Sb<br />
antimony<br />
51<br />
209<br />
Bi<br />
bismuth<br />
83<br />
16<br />
O<br />
oxygen<br />
8<br />
32<br />
S<br />
sulfur<br />
16<br />
79<br />
Se<br />
selenium<br />
34<br />
128<br />
Te<br />
tellurium<br />
52<br />
[209]<br />
Po<br />
polonium<br />
84<br />
19<br />
F<br />
fluorine<br />
9<br />
35.5<br />
Cl<br />
chlorine<br />
17<br />
80<br />
Br<br />
bromine<br />
35<br />
127<br />
I<br />
Iodine<br />
53<br />
[210]<br />
At<br />
astatine<br />
85<br />
4<br />
He<br />
helium<br />
2<br />
20<br />
Ne<br />
neon<br />
10<br />
40<br />
Ar<br />
argon<br />
18<br />
84<br />
Kr<br />
krypton<br />
36<br />
131<br />
Xe<br />
xenon<br />
54<br />
[222]<br />
Rn<br />
radon<br />
86<br />
Key<br />
relative atomic mass<br />
atomic symbol<br />
name<br />
atomic (proton) number<br />
58<br />
Ce<br />
140<br />
90<br />
Th<br />
232<br />
59<br />
Pr<br />
141<br />
91<br />
Pa<br />
231<br />
60<br />
Nd<br />
144<br />
92<br />
U<br />
238<br />
61<br />
Pm<br />
(145)<br />
93<br />
Np<br />
237<br />
62<br />
Sm<br />
150<br />
94<br />
Pu<br />
(244)<br />
63<br />
Eu<br />
152<br />
95<br />
Am<br />
(243)<br />
64<br />
Gd<br />
157<br />
96<br />
Cm<br />
(247)<br />
65<br />
Tb<br />
159<br />
97<br />
Bk<br />
(247)<br />
66<br />
Dy<br />
162<br />
98<br />
Cf<br />
(251)<br />
67<br />
Ho<br />
165<br />
99<br />
Es<br />
(252)<br />
68<br />
Er<br />
167<br />
100<br />
Fm<br />
(257)<br />
69<br />
Tm<br />
169<br />
101<br />
Md<br />
(258)<br />
70<br />
Yb<br />
173<br />
102<br />
No<br />
(259)<br />
71<br />
Lu<br />
175<br />
103<br />
Lr<br />
(260)<br />
TIP<br />
The periodic table shows relative<br />
atomic mass and not mass number.<br />
This is the average mass of the<br />
isotopes of each element.<br />
10