Swissmedic Annual Report 2017: achieving success through collaboration
According to Stéphane Rossini, incoming Chairman of the Agency Council, the culture of collaboration will remain a factor in ensuring that Switzerland is successful in retaining a high-quality medicines control system: “A globalised economy and the international consumption of therapeutic products entail synergies and collaboration.”
According to Stéphane Rossini, incoming Chairman of the Agency Council, the culture of collaboration will remain a factor in ensuring that Switzerland is successful in retaining a high-quality medicines control system: “A globalised economy and the international consumption of therapeutic products entail synergies and collaboration.”
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
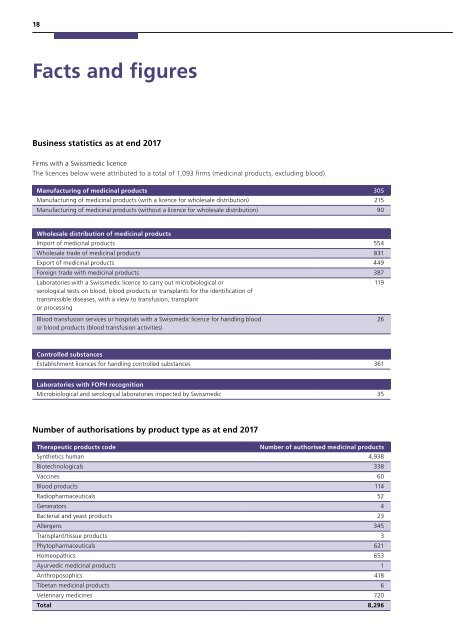
18<br />
Facts and figures<br />
Business statistics as at end <strong>2017</strong><br />
Firms with a <strong>Swissmedic</strong> licence<br />
The licences below were attributed to a total of 1,093 firms (medicinal products, excluding blood).<br />
Manufacturing of medicinal products 305<br />
Manufacturing of medicinal products (with a licence for wholesale distribution) 215<br />
Manufacturing of medicinal products (without a licence for wholesale distribution) 90<br />
Wholesale distribution of medicinal products<br />
Import of medicinal products 554<br />
Wholesale trade of medicinal products 831<br />
Export of medicinal products 449<br />
Foreign trade with medicinal products 387<br />
Laboratories with a <strong>Swissmedic</strong> licence to carry out microbiological or<br />
serological tests on blood, blood products or transplants for the identification of<br />
transmissible diseases, with a view to transfusion, transplant<br />
or processing<br />
Blood transfusion services or hospitals with a <strong>Swissmedic</strong> licence for handling blood<br />
or blood products (blood transfusion activities)<br />
119<br />
26<br />
Controlled substances<br />
Establishment licences for handling controlled substances 361<br />
Laboratories with FOPH recognition<br />
Microbiological and serological laboratories inspected by <strong>Swissmedic</strong> 35<br />
Number of authorisations by product type as at end <strong>2017</strong><br />
Therapeutic products code<br />
Number of authorised medicinal products<br />
Synthetics human 4,938<br />
Biotechnologicals 338<br />
Vaccines 60<br />
Blood products 114<br />
Radiopharmaceuticals 52<br />
Generators 4<br />
Bacterial and yeast products 23<br />
Allergens 345<br />
Transplant/tissue products 3<br />
Phytopharmaceuticals 621<br />
Homeopathics 653<br />
Ayurvedic medicinal products 1<br />
Anthroposophics 418<br />
Tibetan medicinal products 6<br />
Veterinary medicines 720<br />
Total 8,296