Swissmedic Annual Report 2017: achieving success through collaboration
According to Stéphane Rossini, incoming Chairman of the Agency Council, the culture of collaboration will remain a factor in ensuring that Switzerland is successful in retaining a high-quality medicines control system: “A globalised economy and the international consumption of therapeutic products entail synergies and collaboration.”
According to Stéphane Rossini, incoming Chairman of the Agency Council, the culture of collaboration will remain a factor in ensuring that Switzerland is successful in retaining a high-quality medicines control system: “A globalised economy and the international consumption of therapeutic products entail synergies and collaboration.”
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
32 Market access | Marketing authorisation<br />
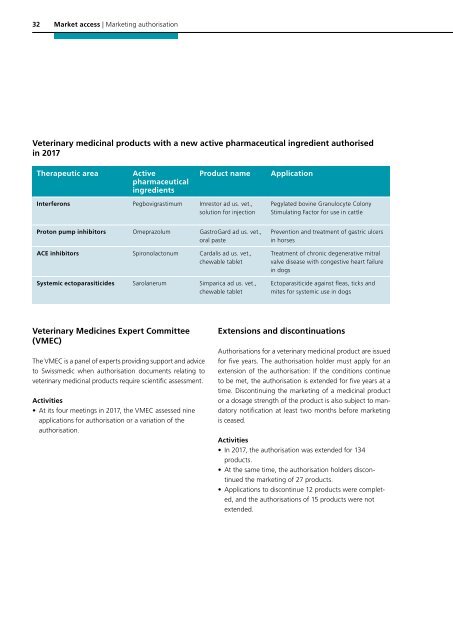
Veterinary medicinal products with a new active pharmaceutical ingredient authorised<br />
in <strong>2017</strong><br />
Therapeutic area<br />
Active<br />
pharmaceutical<br />
ingredients<br />
Product name<br />
Application<br />
Interferons Pegbovigras timum Imrestor ad us. vet.,<br />
solution for injection<br />
Pegylated bovine Granulocyte Colony<br />
Stimulating Factor for use in cattle<br />
Proton pump inhibitors Omeprazolum GastroGard ad us. vet.,<br />
oral paste<br />
ACE inhibitors Spironolactonum Cardalis ad us. vet.,<br />
chewable tablet<br />
Systemic ectoparasiticides Sarolanerum Simparica ad us. vet.,<br />
chewable tablet<br />
Prevention and treatment of gastric ulcers<br />
in horses<br />
Treatment of chronic degenerative mitral<br />
valve disease with congestive heart failure<br />
in dogs<br />
Ectoparasiticide against fleas, ticks and<br />
mites for systemic use in dogs<br />
Veterinary Medicines Expert Committee<br />
(VMEC)<br />
The VMEC is a panel of experts providing support and advice<br />
to <strong>Swissmedic</strong> when authorisation documents relating to<br />
veterinary medicinal products require scientific assessment.<br />
Activities<br />
• At its four meetings in <strong>2017</strong>, the VMEC assessed nine<br />
applications for authorisation or a variation of the<br />
authorisation.<br />
Extensions and discontinuations<br />
Authorisations for a veterinary medicinal product are issued<br />
for five years. The authorisation holder must apply for an<br />
extension of the authorisation: If the conditions continue<br />
to be met, the authorisation is extended for five years at a<br />
time. Discontinuing the marketing of a medicinal product<br />
or a dosage strength of the product is also subject to mandatory<br />
notification at least two months before marketing<br />
is ceased.<br />
Activities<br />
• In <strong>2017</strong>, the authorisation was extended for 134<br />
products.<br />
• At the same time, the authorisation holders discontinued<br />
the marketing of 27 products.<br />
• Applications to discontinue 12 products were completed,<br />
and the authorisations of 15 products were not<br />
extended.