Chemistry and Chemical Physics Graduate Programs brochure
Chemistry and Chemical Physics Graduate Programs brochure
Chemistry and Chemical Physics Graduate Programs brochure
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
BENJAMIN T. KING<br />
Associate Professor<br />
Organic <strong>Chemistry</strong><br />
E-mail: king@unr.edu<br />
24 - Faculty<br />
B.S. (1992), Northeastern University; Ph.D. (2000),<br />
University of Colorado (J. Michl); NIH Postdoctoral<br />
Fellow (2000-02), University of California, Berkeley<br />
(R.G. Bergman).<br />
Our research focuses on the preparation of molecules that might someday<br />
serve as useful materials. The approach is to design synthetic targets using<br />
computational chemistry, prepare them by chemical synthesis, <strong>and</strong> then<br />
study their properties <strong>and</strong> behavior.<br />
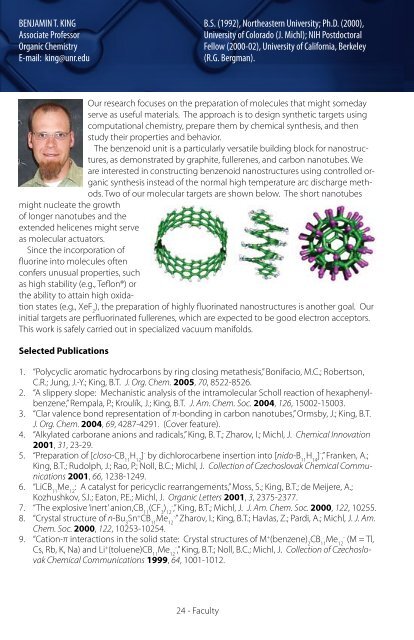
The benzenoid unit is a particularly versatile building block for nanostructures,<br />
as demonstrated by graphite, fullerenes, <strong>and</strong> carbon nanotubes. We<br />
are interested in constructing benzenoid nanostructures using controlled organic<br />
synthesis instead of the normal high temperature arc discharge methods.<br />
Two of our molecular targets are shown below. The short nanotubes<br />
might nucleate the growth<br />
of longer nanotubes <strong>and</strong> the<br />
extended helicenes might serve<br />
as molecular actuators.<br />
Since the incorporation of<br />
fluorine into molecules often<br />
confers unusual properties, such<br />
as high stability (e.g., Teflon®) or<br />
the ability to attain high oxidation<br />
states (e.g., XeF 2 ), the preparation of highly fluorinated nanostructures is another goal. Our<br />
initial targets are perfluorinated fullerenes, which are expected to be good electron acceptors.<br />
This work is safely carried out in specialized vacuum manifolds.<br />
Selected Publications<br />
1. “Polycyclic aromatic hydrocarbons by ring closing metathesis,” Bonifacio, M.C.; Robertson,<br />
C.R.; Jung, J.-Y.; King, B.T. J. Org. Chem. 2005, 70, 8522-8526.<br />
2. “A slippery slope: Mechanistic analysis of the intramolecular Scholl reaction of hexaphenylbenzene,”<br />
Rempala, P.; Kroulík, J.; King, B.T. J. Am. Chem. Soc. 2004, 126, 15002-15003.<br />
3. “Clar valence bond representation of π-bonding in carbon nanotubes,” Ormsby, J.; King, B.T.<br />
J. Org. Chem. 2004, 69, 4287-4291. (Cover feature).<br />
4. “Alkylated carborane anions <strong>and</strong> radicals,” King, B. T.; Zharov, I.; Michl, J. <strong>Chemical</strong> Innovation<br />
2001, 31, 23-29.<br />
5. “Preparation of [ closo-CB H ] 11 12 - by dichlorocarbene insertion into [nido-B H ] 11 14 - ,” Franken, A.;<br />
King, B.T.; Rudolph, J.; Rao, P.; Noll, B.C.; Michl, J. Collection of Czechoslovak <strong>Chemical</strong> Communications<br />
2001, 66, 1238-1249.<br />
6. “LiCB11Me<br />
: A catalyst for pericyclic rearrangements,” Moss, S.; King, B.T.; de Meijere, A.;<br />
12<br />
Kozhushkov, S.I.; Eaton, P.E.; Michl, J. Organic Letters 2001, 3, 2375-2377.<br />
7. “The explosive ‘inert’ anion,CB11<br />
(CF3 ) 8.<br />
- ,” King, B.T.; Michl, J. J. Am. Chem. Soc. 2000, 122, 10255.<br />
12<br />
“Crystal structure of n-Bu Sn 3 + -, CB Me ” Zharov, I.; King, B.T.; Havlas, Z.; Pardi, A.; Michl, J. J. Am.<br />
11 12<br />
Chem. Soc. 2000, 122, 10253-10254.<br />
9.<br />
+ - “Cation-π interactions in the solid state: Crystal structures of M (benzene)2CB Me (M = Tl,<br />
11 12<br />
Cs, Rb, K, Na) <strong>and</strong> Li + - (toluene)CB Me ,” King, B.T.; Noll, B.C.; Michl, J. Collection of Czechoslo-<br />
11 12<br />
vak <strong>Chemical</strong> Communications 1999, 64, 1001-1012.