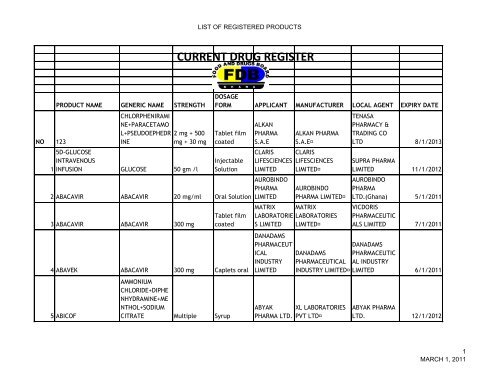
CURRENT DRUG REGISTER
CURRENT DRUG REGISTER
CURRENT DRUG REGISTER
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
NO 123<br />
1<br />
PRODUCT NAME GENERIC NAME STRENGTH<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PSEUDOEPHEDR<br />
INE<br />
2 mg + 500<br />
mg + 30 mg<br />
5D-GLUCOSE<br />
INTRAVENOUS<br />
INFUSION GLUCOSE 50 gm /l<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
DOSAGE<br />
FORM APPLICANT MANUFACTURER LOCAL AGENT EXPIRY DATE<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
2 ABACAVIR ABACAVIR 20 mg/ml Oral Solution<br />
3 ABACAVIR ABACAVIR 300 mg<br />
Tablet film<br />
coated<br />
4 ABAVEK ABACAVIR 300 mg Caplets oral<br />
5 ABICOF<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE+ME<br />
NTHOL+SODIUM<br />
CITRATE Multiple Syrup<br />
<strong>CURRENT</strong> <strong>DRUG</strong> <strong>REGISTER</strong><br />
ALKAN<br />
PHARMA<br />
S.A.E<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
ALKAN PHARMA<br />
S.A.E<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
ABYAK<br />
PHARMA LTD.<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
TENASA<br />
PHARMACY &<br />
TRADING CO<br />
LTD 8/1/2013<br />
SUPRA PHARMA<br />
LIMITED 11/1/2012<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 6/1/2011<br />
ABYAK PHARMA<br />
LTD. 12/1/2012<br />
1<br />
MARCH 1, 2011
6 ABYCAM PIROXICAM 20 mg<br />
7 ABYCOLD<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PHENYLEPHRIN<br />
E+OTHERS<br />
8 ABYDIUM LOPERAMIDE 2 mg<br />
9 ABYPAIN<br />
10 ABYVITA<br />
11 ABYVITA<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
500 mg/50<br />
mg Caplets oral<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
500 mg/50<br />
mg Tablet Oral<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
12 ACEM CLARITHROMYCIN 250 mg Tablet Oral<br />
13 ACETYLCYSTEINE ACETYLCYSTEINE 1 g/5 ml<br />
14 A-CIP -D<br />
Injectable<br />
Solution<br />
CIPROFLOXACIN+<br />
DEXAMETHASONE 0.3% + 0.1% Eye drops<br />
15 ACLOTAS ACECLOFENAC 100 mg Tablet Oral<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
AMBICA<br />
PHARMA<br />
SALES<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
ABYAK PHARMA<br />
LTD. 3/1/2012<br />
ABYAK PHARMA<br />
LTD. 12/31/2012<br />
ABYAK PHARMA<br />
LTD. 3/1/2012<br />
ABYAK PHARMA<br />
LTD. 12/31/2012<br />
ABYAK PHARMA<br />
LTD. 12/31/2012<br />
ABYAK PHARMA<br />
LTD. 11/1/2013<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 6/1/2013<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
AMBICA PHARMA<br />
SALES<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD -DEHRADUN<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
SPINTEX<br />
CHEMIST LTD 12/31/2012<br />
GR PHARMA<br />
LTD. 12/1/2013<br />
2<br />
MARCH 1, 2011
16 ACNE AID<br />
SULPHATED<br />
SURFACTANT<br />
BLEND 6.3% Solid Soap<br />
17 ACNE FREE TRETINOIN 0.05 %w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
18 ACNE FREE TRETINOIN 0.02 %w/w Gel Topical<br />
19 ACNOTIN ISOTRETINOIN 20 mg<br />
20 ACNOTIN ISOTRETINOIN 10 mg<br />
21 ACRIFLAVINE ACRIFLAVINE 0.5%<br />
22 ACTIFED<br />
23 ACTIFED<br />
24<br />
ACTIFED<br />
EXPECTORANT<br />
Capsule oral<br />
(Softgel)<br />
Capsule oral<br />
(Softgel)<br />
Topical<br />
Lotion<br />
PSEUDOEPHEDRIN<br />
E+TRIPROLIDINE Multiple Syrup<br />
PSEUDOEPHEDRIN<br />
E+TRIPROLIDINE Multiple Tablet Oral<br />
GUAIPHENESIN+PS<br />
EUDOEPHEDRINE+<br />
TRIPOLIDINE Multiple Syrup<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S(UK) LABORATORIES<br />
LIMITED (IRELAND) LTD<br />
AMOUN<br />
PHARMACEUT<br />
ICALS<br />
AMOUN<br />
PHARMACEUT<br />
ICALS<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
PARKERSTEIN<br />
(GH) LTD 3/1/2012<br />
AMOUN<br />
PHARMACEUTICALS REHA MEDICAL<br />
SUPPLY 2/1/2013<br />
AMOUN<br />
PHARMACEUTICALS REHA MEDICAL<br />
SUPPLY 2/1/2013<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
KAMA INDUSTRIES<br />
LTD.<br />
GLAXOWELCOME<br />
EGYPT, S.A.E.<br />
GLAXOWELCOME<br />
EGYPT, S.A.E.<br />
GLAXOWELCOME<br />
EGYPT, S.A.E.<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD 10/1/2012<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD 9/17/2014<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2012<br />
3<br />
MARCH 1, 2011
25<br />
ACTIVATED<br />
CHARCOAL<br />
26 ACTOPHLEM<br />
ACTIVATED<br />
CHARCOAL 50 g<br />
AMMOMIUM+DIPH<br />
ENYLPYRALINE<br />
HCL+ETOFYLLINE<br />
+SODIUM<br />
CITRATE+THEO Multiple Syrup<br />
27 ACTRAPID HM HUMAN INSULIN 100 iu/10 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral Solution<br />
Injectable<br />
Suspension<br />
28 ACYCLOVIR ACICLOVIR 200 mg Tablet Oral<br />
29 ACYCLOVIR ACICLOVIR 200 mg Tablet Oral<br />
30 ACYCLOVIR DENK ACICLOVIR 200 mg Tablet Oral<br />
31 ACYCLOVIR DENK ACICLOVIR 5%w/w<br />
Cream<br />
Topical<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD HIPLUS,S.A<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
ALDAPH SPA<br />
(NOVO<br />
NORDISK<br />
ALGERIA)<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
PHARMA-<br />
Q(Pty)Ltd.<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
ALDAPH SPA (NOVO<br />
NORDISK<br />
ALGERIA) GOKALS LTD 4/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
4<br />
MARCH 1, 2011
32 ADAMSUNATE ARTESUNATE 50 mg Caplets oral<br />
33 ADERON<br />
34 ADMAG<br />
VITAMIN<br />
A+VITAMIN D3<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
5000 iu/500<br />
iu<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Softgel)<br />
570 mg/300<br />
mg/60 mg/15<br />
mls Syrup<br />
35 ADRENALINE ADRENALINE 1.0 mg/ml<br />
36 ADRENALINE ADRENALINE 1.0 mg/ml<br />
37 ADRENALINE ADRENALINE 1.0 mg/ml<br />
38<br />
ADRENALINE &<br />
LIGNOCAINE<br />
39 ADULT-KOFOF<br />
ADRENALINE+LIGN<br />
OCAINE 2%+50 mg/ml<br />
DEXTROMETHORP<br />
HAN+GUAIFENISIN<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
10 mg/50<br />
mg/5 mls Syrup<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
T.P. <strong>DRUG</strong><br />
LABORATORIES<br />
(1969) CO., LTD<br />
VAST FAVOUR<br />
INTERNATIONAL<br />
LTD<br />
DANDONG<br />
PHARMACEUTIC<br />
AL FACTORY 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 3/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
TOBINCO<br />
PHARMACY 10/1/2011<br />
5<br />
MARCH 1, 2011
40 ADVANTAN<br />
METHYLPREDNISO<br />
LONE 1%w/w<br />
41 AERIUS DESLORATIDINE 5 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Tablet film<br />
coated<br />
42 AERIUS DESLORATIDINE 0.5 mg/ml Syrup<br />
43 AIRTAL ACECLOFENAC 100 mg<br />
44 AKES-N-PAINS<br />
45 ALAXIN<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
DIHYDROARTEMISI<br />
NIN 40 mg/2mls<br />
Tablet film<br />
coated<br />
50 mg + 500<br />
mg Tablet Oral<br />
46 ALAZOLE ALBENDAZOLE 400 mg Tablet Oral<br />
47 ALBEFAR ALBENDAZOLE 200 mg/5 mls<br />
48 ALBEFAR ALBENDAZOLE 400 mg<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
ALMIRALL<br />
PRODESFARM<br />
A, S.L.<br />
PERFECT<br />
PHARMACEUT<br />
ICALS LTD<br />
Injectable<br />
Solution GVS LABS<br />
Oral<br />
Suspension<br />
Tablet<br />
Chewable<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
BAYER - SCHERING<br />
PHARMA<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
SCHERING-PLOUGH<br />
LABO<br />
N.V.(BELGIUM)SC<br />
HERING-PLOUGH<br />
S.p. A. (ITALY) GOKALS LTD 12/31/2011<br />
SCHERING-PLOUGH<br />
LABO<br />
N.V.(BELGIUM) GOKALS LTD 12/31/2011<br />
ALMIRALL<br />
PRODESFARMA,<br />
S.L.<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
PERFECT<br />
PHARMACEUTICALS<br />
LTD 7/1/2013<br />
KILITCH <strong>DRUG</strong>S<br />
(INDIA) LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
STERLING<br />
LAB STERLING LAB<br />
STERLING<br />
LAB STERLING LAB<br />
TOBINCO<br />
PHARMACY 11/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 12/31/2012<br />
FAR EAST<br />
MERCANTILE 7/1/2013<br />
FAR EAST<br />
MERCANTILE 7/1/2013<br />
6<br />
MARCH 1, 2011
49 ALBENDAZOLE ALBENDAZOLE 200 mg/5ml<br />
50 ALBENDAZOLE ALBENDAZOLE 400 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Tablet<br />
Chewable<br />
51 ALDACTONE SPIRONOLACTONE 25 mg Tablet Oral<br />
52 ALDACTONE SPIRONOLACTONE 100 mg Tablet Oral<br />
53 ALDOMET METHYLDOPA 250 mg<br />
Tablet film<br />
coated<br />
54 ALERID CETIRIZINE 10 mg Tablet Oral<br />
55 ALESOF CETIRIZINE 10 mg<br />
56 ALL CORNERS SULPHUR<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
5<br />
teaspoon/bat Powder for<br />
ch<br />
topical use<br />
57 ALLOPURINOL ALLOPURINOL 100 mg Tablet Oral<br />
58 ALLOPURINOL ALLOPURINOL 300 mg Tablet Oral<br />
COLORAMA<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
ROCK<br />
CHEMISTS<br />
OSAKA PHARMA<br />
PVT. LTD.<br />
TOBINCO<br />
PHARMACY 2/1/2012<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
SEARLE DIVISON OF<br />
MONSANTO plc.<br />
(UK)PHARMACIA &<br />
UPJOHN INT GOKALS LTD 12/31/2011<br />
SEARLE DIVISON OF<br />
MONSANTO plc.<br />
(UK)PHARMACIA &<br />
UPJOHN INT GOKALS LTD 12/31/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 10/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
XL LABORATORIES<br />
PVT LTD<br />
ALL CORNERS<br />
LTD ALL CORNERS LTD<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
ALL CORNERS<br />
LTD 12/31/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
7<br />
MARCH 1, 2011
59 ALLOPURINOL ALLOPURINOL 100 mg Tablet Oral<br />
60 ALLOPURINOL ALLOPURINOL 300 mg Tablet Oral<br />
61 ALLOPURINOL ALLOPURINOL 300 mg Tablet Oral<br />
62 ALLOPURINOL ALLOPURINOL 100 mg Tablet Oral<br />
63 ALLOPURINOL ALLOPURINOL 100 mg Tablet Oral<br />
64 ALLOPURINOL ALLOPURINOL 300 mg Tablet Oral<br />
65 ALOMIDE<br />
66<br />
LODOXAMIDE<br />
TROMETHAMINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
1.78 mg/1<br />
mg Eye drops<br />
ALPHA AND OMEGA<br />
LINIMENT Liniment<br />
67 ALTHROCIN S ERYTHROMYCIN 500 mg Tablet Oral<br />
ROCK<br />
CHEMISTS<br />
ROCK<br />
CHEMISTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ALCON-<br />
COUVREUR<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ALCON-<br />
COUVREUR<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ALCON-<br />
COUVREUR 9/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
DOVE<br />
PHARMACY<br />
LIMITED 10/1/2012<br />
8<br />
MARCH 1, 2011
68<br />
69<br />
ALUMINIUM<br />
HYDROXIDE<br />
ALUMINIUM<br />
HYDROXIDE<br />
70 ALUSIL<br />
71 ALVITE<br />
72 AMADAY<br />
73 AMADAY<br />
ALUMINIUM<br />
HYDROXIDE 500 mg Tablet Oral<br />
ALUMINIUM<br />
HYDROXIDE 500 mg Tablet Oral<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
TRISILICATE<br />
250 mg/500<br />
mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Chewable<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup GVS LABS GVS LABS<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
74 AMARYL GLIMEPIRIDE 4 mg Tablet Oral<br />
75 AMARYL GLIMEPIRIDE 2 mg Tablet Oral<br />
76 AMARYL GLIMEPIRIDE 1 mg Tablet Oral<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
AVENTIS<br />
PHARMA<br />
DEUTSCHLAN<br />
D<br />
AVENTIS<br />
PHARMA<br />
DEUTSCHLAN<br />
D<br />
AVENTIS<br />
PHARMA<br />
DEUTSCHLAN<br />
D<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
AVENTIS PHARMA<br />
DEUTSCHLANDAVE<br />
NTIS PHARMA<br />
S.p.A(Italy) GOKALS LTD 9/1/2013<br />
AVENTIS PHARMA<br />
DEUTSCHLANDAVE<br />
NTIS PHARMA<br />
S.p.A(Italy) GOKALS LTD 8/1/2013<br />
AVENTIS PHARMA<br />
DEUTSCHLANDAVE<br />
NTIS PHARMA<br />
S.p.A(Italy) GOKALS LTD 8/1/2013<br />
9<br />
MARCH 1, 2011
77 AMARYL GLIMEPIRIDE 3 mg Tablet Oral<br />
78 AMBESYL 10<br />
79 AMBESYL 5<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
80 AMBICORT HYDROCORTISONE 100 mg<br />
81 AMBROLITE-GS<br />
82 AMCLAV<br />
83 AMCLOX<br />
84 AMCOF COUGH<br />
AMBROXOL+GUAIF<br />
ENESIN+SALBUTA<br />
MOL<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
30 mg/2<br />
mg/50 mg/5<br />
mls Syrup<br />
500 mg/125<br />
mg<br />
AMPICILLIN+CLOX<br />
ACILLIN 250, 250 mg<br />
Tablet film<br />
coated<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
SANOFI<br />
AVENTIS<br />
NIGERIA<br />
LIMITED<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
AMBICA<br />
PHARMA<br />
SALES<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
SANOFI-AVENTIS<br />
S.p.A<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
AMBICA PHARMA<br />
SALES<br />
TABLETS (INDIA)<br />
LIMITED<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
AMMONIUM<br />
CHLORIDE+CHLOR<br />
PHENIRAMINE+EP<br />
HEDRINE+SODIUM<br />
CITRATE Multiple Syrup NIC PHARMA<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
MARVI<br />
LABORATORIES<br />
GOKALS-<br />
LABOREX LTD 8/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 11/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 11/1/2013<br />
SPINTEX<br />
CHEMIST LTD 8/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 4/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 8/1/2012<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 8/1/2013<br />
SALOM<br />
PHARMACY<br />
LIMITED 10/1/2011<br />
10<br />
MARCH 1, 2011
85 AMCOLEX-500<br />
86 AMIDERM<br />
AMPICILLIN+CLOX<br />
ACILLIN<br />
250 mg + 250<br />
mg<br />
GENTAMYCIN+BET<br />
AMETHASONE+TO<br />
LNAFTATE+CLIOQ<br />
UINOL Multiple<br />
87 AMIKACIN AMIKACIN 500 mg<br />
88 AMILIP-10<br />
89 AMILIP-5<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Cream<br />
Topical<br />
Injectable<br />
Powder<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
90 AMINOPHYLLINE AMINOPHYLLINE 25 mg/ml<br />
91 AMINOPHYLLINE AMINOPHYLLINE 25 mg/ml<br />
92 AMINOPHYLLINE AMINOPHYLLINE 25 mg/ml<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
93 AMIODARONE AMIODARONE 200 mg Tablet Oral<br />
94 AMIODARONE AMIODARONE 200 mg Tablet Oral<br />
UNICHEM<br />
INDUSTRIES<br />
LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
ROCK<br />
CHEMISTS<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
UNICHEM GHANA<br />
LTD.<br />
MISSION VIVACARE<br />
LIMITED<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
GB PHARMA<br />
(GH) LTD 8/1/2011<br />
DEVATS INDIA PVT<br />
LTD ROCK CHEMISTS 2/1/2012<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 3/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
ROCK<br />
CHEMISTS<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK)<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
11<br />
MARCH 1, 2011
95 AMITONE<br />
96<br />
97<br />
98<br />
99<br />
100<br />
101<br />
102<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup GVS LABS GVS LABS<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 25 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 25 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 50 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 25 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 25 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 25 mg Tablet Oral<br />
AMITRIPTYLLINE<br />
HYDROCHLORIDE AMITRIPTYLINE 50 mg Tablet Oral<br />
103 AMLO<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
ROCK<br />
CHEMISTS<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
BRAVO<br />
HEALTHCARE<br />
LTD<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
BRAVO<br />
HEALTHCARE LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
GLOBAL<br />
BIOPHARMACEU<br />
TICALS LTD 11/1/2012<br />
12<br />
MARCH 1, 2011
104 AMLOCOR-10<br />
105 AMLOCOR-5<br />
106 AMLODAC-AT<br />
107 AMLO-DENK<br />
108 AMLO-DENK<br />
109 AMLODIPINE<br />
110 AMLODIPINE<br />
111 AMLONOVA<br />
112 AMLONOVA<br />
113 AMLOPRESS<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
ATENOLOL+AMLO<br />
DIPINE 50 mg/10 mg Tablet Oral<br />
AMLODIPINE<br />
MESILATE 5 mg Tablet Oral<br />
AMLODIPINE<br />
MESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 4/1/2011<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 4/1/2011<br />
MADRAS<br />
PHARMACEUTICALS<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOVA<br />
LTD.<br />
PHARMANOVA<br />
LTD.<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
GOKALS-<br />
LABOREX LTD 12/1/2011<br />
GOKALS-<br />
LABOREX LTD 9/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 11/1/2012<br />
13<br />
MARCH 1, 2011
114 AMLOPRESS<br />
115 AMLOPRIL<br />
116 AMOKSIKLAV<br />
117 AMOKSIKLAV<br />
118 AMOKSIKLAV 600 mg<br />
119 AMOSARC<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE+LISIN<br />
OPRIL 10 mg/10 mg Tablet Oral<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 1 g/0.2 g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 1000 mg/1g Tablet Oral<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
500 mg/100<br />
mg<br />
Injectable<br />
Powder<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
120 AMOWIN 10 AMLODIPINE 10 mg Tablet Oral<br />
121 AMOXICILLIN AMOXICILLIN 250 mg<br />
122 AMOXICILLIN AMOXICILLIN 250 mg<br />
123 AMOXICILLIN AMOXICILLIN 125 mg/5ml<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Powder for<br />
Oral<br />
Suspension<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 11/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
PALB<br />
PHARMACEUT<br />
ICALS<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
PALB<br />
PHARMACEUT<br />
ICALS<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
BELCO<br />
PHARMA PVT<br />
LTD<br />
MADRAS<br />
PHARMACEUTICALS<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
MISSION VIVACARE<br />
LIMITED<br />
BELCO PHARMA<br />
PVT LTD<br />
TROGE<br />
MEDICAL<br />
GMBH MEDOPHARM<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 4/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 2/24/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 4/1/2013<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
HILLS<br />
PHARMACY 12/1/2012<br />
UNICHEM<br />
GHANA LTD. 11/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
14<br />
MARCH 1, 2011
124 AMOXICILLIN AMOXICILLIN 250 mg/5 ml<br />
125<br />
AMOXI-DENK<br />
CHEWABLE AMOXICILLIN 1000 mg<br />
126 AMOXI-DENK DISPERS AMOXICILLIN 250 mg<br />
127 AMOXIL AMOXICILLIN 250 mg<br />
128 AMOXIL AMOXICILLIN 125 mg/ 5ml<br />
129 AMOXYCILLIN AMOXICILLIN 500 mg<br />
130 AMOXYLEX AMOXICILLIN 125 mg/5 ml<br />
131 AMPICILLIN AMPICILLIN 500 mg<br />
132 AMPICILLIN AMPICILLIN 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Tablet<br />
Chewable<br />
Tablet<br />
Dispersible<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Powder for<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG CIMEX AG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG CIMEX AG<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
UNICHEM<br />
INDUSTRIES<br />
LTD<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
GLAXOSMITHKLINE<br />
INTERNATIONAL<br />
MEDREICH<br />
LIMITEDGLAXOSMI<br />
THKLINE<br />
INTERNATIONAL<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
UNICHEM<br />
INDUSTRIES LTD<br />
TROGE MEDICAL<br />
GMBH<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
KINAPHARMA<br />
LTD 11/4/2014<br />
GOKALS-<br />
LABOREX LTD 7/1/2011<br />
GOKALS-<br />
LABOREX LTD 7/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 12/31/2012<br />
UNICHEM<br />
GHANA LTD. 6/1/2011<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
15<br />
MARCH 1, 2011
133 AMPICILLIN AMPICILLIN 250 mg<br />
134<br />
AMPICILLIN +<br />
CLOXACILLIN<br />
AMPICILLIN+CLOX<br />
ACILLIN<br />
135 AMPICILLIN SODIUM AMPICILLIN 0.5 g<br />
250 mg + 250<br />
mg<br />
136 AMPIN AMPICILLIN 500 mg<br />
137 AMTAS<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
138 A-MYCIN AZITHROMYCIN 200 mg/5 ml<br />
139 AMZAK OD<br />
140 AMZAK OD<br />
141 ANAFEN FORTE<br />
Oral<br />
Suspension<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
325 mg + 400<br />
mg Tablet Oral<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
AMBICA<br />
PHARMA<br />
SALES<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
GR PHARMA<br />
LTD. 10/1/2011<br />
SPINTEX<br />
CHEMIST LTD 8/1/2013<br />
FAR EAST<br />
MERCANTILE 9/1/2011<br />
FAR EAST<br />
MERCANTILE 9/1/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 2/1/2013<br />
16<br />
MARCH 1, 2011
142 ANAFLAM<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
143 ANAFRANIL CLOMIPRAMINE 25 mg<br />
144 ANALGIN METAMiZOLE 500 mg/2 ml<br />
145 ANAPAIN EXTRA<br />
146<br />
147<br />
ANDREWS LIVER SALT<br />
(LEMON)<br />
ANDREWS LIVER SALT<br />
(ORIGINAL)<br />
CAFFEINE+PARAC<br />
ETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg/400<br />
mg Tablet Oral<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
500 mg/30<br />
mg Tablet Oral<br />
SODIUM<br />
BICARBONATE+SO<br />
DIUM CARBONATE 2.29 g/0.50 g<br />
MAGNESIUM<br />
SULPHATE+SODIU<br />
M<br />
CARBONATE+SODI<br />
UM BICARBONATE<br />
0.88<br />
g/0.1g/2.18<br />
g<br />
Powder for<br />
Oral Solution<br />
Powder for<br />
Oral Solution<br />
148 ANFEN IBUPROFEN 200 mg Tablet Oral<br />
SHALINA<br />
LABORATORIE<br />
S PVT. LTD.<br />
NOVARTIS<br />
FARMA SPA<br />
(ITALY)<br />
SINOPHARM<br />
GH LTD<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
FREDUN<br />
PHARMACEUTICALS<br />
LTDSHALINA<br />
LABORATORIES<br />
PVT. LTD.<br />
NOVARTIS PHARMA<br />
AG<br />
CHINA NATIONAL<br />
PHARM. FOREIGN<br />
TRADE CORP.<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
GLAXOSMITHKLINE<br />
COSTA RICA S.A.<br />
SOCOMEX<br />
PHARMACY LTD. 10/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
ALOKEN<br />
PHARMACY 12/20/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 8/1/2013<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLINE<br />
COSTA RICA S.A. ROCK CHEMISTS 4/1/2011<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 7/1/2012<br />
17<br />
MARCH 1, 2011
149 ANFEN IBUPROFEN 400 mg Tablet Oral<br />
150 ANTEL ALBENDAZOLE 400.00 mg Caplets oral<br />
151 ANTYCOLD-R<br />
152 APCAN PLUS<br />
153 APCOLIN<br />
154 APETI<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL+PHE<br />
NYLEPHRINE<br />
ADRENALINE+LIGN<br />
OCAINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg+4.00<br />
mg+500 mg+<br />
2.5 mg Tablet Oral<br />
2% (20mg +<br />
0.005mg)<br />
SUXAMETHONIUM<br />
CHLORIDE 50 mg<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
155 APNAC DICLOFENAC 1%w/w Gel Topical<br />
156 APRESOLINE HYDRALAZINE 25 mg<br />
Tablet film<br />
coated<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
AMBICA<br />
PHARMA<br />
SALES<br />
AMBICA<br />
PHARMA<br />
SALES<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
AMBICA<br />
PHARMA<br />
SALES<br />
NOVARTIS<br />
PHARMA AG<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANDONG<br />
PHARMACEUTICAL<br />
FACTORY<br />
SOCOMED PHARMA<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
GEO MEDICORE<br />
LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD<br />
(UK)NOVARTIS<br />
URUNLERI<br />
(TURKEY)<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 7/1/2012<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 7/1/2012<br />
HOCHIEZ<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2011<br />
SPINTEX<br />
CHEMIST LTD 4/1/2013<br />
SPINTEX<br />
CHEMIST LTD 6/1/2013<br />
GEO MEDICORE<br />
LIMITED 5/1/2012<br />
SPINTEX<br />
CHEMIST LTD 9/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 3/1/2013<br />
18<br />
MARCH 1, 2011
157 APRESOLINE HYDRALAZINE 20 mg<br />
158 AQUEOUS CREAM BP<br />
159 AQUEOUS CREAM BP<br />
160 ARBI FORTE<br />
EMULSIFYING<br />
OINTMENT 30 %w/w<br />
EMULSIFYING<br />
OINTMENT 30 %w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
AMINO<br />
ACIDS+CYPROHEP<br />
TADINE+VITAMINS Multiple Syrup<br />
161 ARCALION SALBUTIAMINE 200 mg<br />
162 ARCOMETHER ARTEMETHER 80 mg/ml<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
163 ARIMIDEX ANASTROZOLE 1 mg Tablet Oral<br />
164 ARIXON I.M.<br />
CEFTRIAXONE+LIG<br />
NOCAINE<br />
1000 mg/1<br />
%w/v<br />
Injectable<br />
Solution<br />
NOVARTIS<br />
PHARMA AG<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD<br />
(UK)NOVARTIS<br />
URUNLERI<br />
(TURKEY)<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNI-MED<br />
INDIA UNI-MED INDIA<br />
SERVIER<br />
EGYPT<br />
INDUSTRY<br />
LTD<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
ASTRAZENEC<br />
A UK LTD<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SERVIER EGYPT<br />
INDUSTRY LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
ASTRAZENECA<br />
AB(Sweden)<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITEDFISONS<br />
(BANGLADESH) LTD<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 3/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
VICTORY<br />
PHARMACY 4/1/2011<br />
OYSTER<br />
HEALTHCARE<br />
LTD. 4/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 7/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 2/1/2012<br />
HILLS<br />
PHARMACY 9/18/2011<br />
19<br />
MARCH 1, 2011
165 ARIXON IM<br />
CEFTRIAXONE+LIG<br />
NOCAINE<br />
500 mg/1<br />
%w/v<br />
166 AROMASIN EXEMASTANE 25 mg<br />
167 ARSUAMOON<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
168 ARTEERON ARTEMETHER 80 mg<br />
169 ARTEFAN 20/120<br />
170 ARTEM<br />
171 ARTEM<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
BETA-<br />
ARTEMETHER 80 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Tablet film<br />
coated<br />
150 mg/50<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
20 mg+ 120<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
BETA-<br />
Injectable<br />
ARTEMETHER 40 mg/0.5 ml Solution<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
GUILIN<br />
PHARMACEUT<br />
ICALS CO.<br />
LTD.<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
AJANTA<br />
PHARMA<br />
(INDIA) LTD.<br />
KUNMING<br />
PHARMACEUT<br />
ICAL CORP.<br />
KUNMING<br />
PHARMACEUT<br />
ICAL CORP.<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITEDFISONS<br />
(BANGLADESH) LTD<br />
PFIZER ITALIA S.r.<br />
L.<br />
CHINA NATIONAL<br />
PHARM. FOREIGN<br />
TRADE CORP.<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
AJANTA PHARMA<br />
(INDIA) LTD.<br />
KUNMING<br />
PHARMACEUTICAL<br />
CORP.<br />
KUNMING<br />
PHARMACEUTICAL<br />
CORP.<br />
HILLS<br />
PHARMACY 9/1/2011<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 11/1/2012<br />
SINOPHARM GH<br />
LTD 10/1/2013<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 6/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 4/1/2012<br />
REHA MEDICAL<br />
SUPPLY 6/1/2013<br />
REHA MEDICAL<br />
SUPPLY 6/1/2013<br />
20<br />
MARCH 1, 2011
172<br />
173<br />
174<br />
ARTEMAL COMPLEX(0-<br />
6yrs)<br />
ARTEMAL COMPLEX(7-<br />
13yrs)<br />
ARTEMAL<br />
COMPLEX(Adults)<br />
175 ARTENAM<br />
176 ARTENAM<br />
177<br />
178<br />
ARTESUNATE +<br />
AMODIAQUINE<br />
ARTESUNATE/<br />
AMODIAQUINE<br />
HYDROCHLORIDE<br />
179 ARTETRINE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
BETA-<br />
ARTEMETHER 40 mg/2 ml<br />
BETA-<br />
ARTEMETHER 100mg/ml<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg/200<br />
mg Tablet Oral<br />
50 mg/200<br />
mg Tablet Oral<br />
50 mg/200<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
153.1 mg +<br />
50 mg Tablet Oral<br />
50 mg/ 150<br />
mg Tablet Oral<br />
20 mg + 120<br />
mg Tablet Oral<br />
LACHIFARMA<br />
S.R.L<br />
LACHIFARMA<br />
S.R.L<br />
LACHIFARMA<br />
S.R.L<br />
LACHIFARMA<br />
S.R.L<br />
LACHIFARMA<br />
S.R.L<br />
LACHIFARMA<br />
S.R.L<br />
ARENCO<br />
PHARMACEUT<br />
ICA NV EBEWE PHARMA<br />
ARENCO<br />
PHARMACEUT<br />
ICA NV EBEWE PHARMA<br />
IDA<br />
FOUNDATION IPCA LABS. LTD.<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
POKU<br />
PHARMA LTD.<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
PHARMANOVA<br />
LTD.<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 12/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 12/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 12/1/2012<br />
HEM PHARMA<br />
SERVICES<br />
LIMITED 12/1/2012<br />
HEM PHARMA<br />
SERVICES<br />
LIMITED 12/1/2012<br />
HEALTH ACCESS<br />
NETWORK 9/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
POKU PHARMA<br />
LTD. 4/1/2011<br />
21<br />
MARCH 1, 2011
180 ARTHROTEC<br />
181 ARTRIFAN TABLETS<br />
182 ARTUQUIN<br />
183 ARTUQUIN<br />
184 ARUNATE-AQ<br />
185 ASCAP<br />
186 ASCOLD<br />
DICLOFENAC+MIS<br />
OPROSTOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg/ 200<br />
mcg Tablet Oral<br />
ARTEMETHER+LU<br />
MEFANTRINE 20/120 mg Tablet Oral<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
ASPIRIN +<br />
CAFFEINE+<br />
PARACETAMOL<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL+PHE<br />
NYLEPHRINE<br />
100 mg/300<br />
mg Tablet Oral<br />
50 mg/150<br />
mg<br />
Tablet<br />
Dispersible<br />
153 mg/50<br />
mg Tablet Oral<br />
150 mg+30<br />
mg+250 mg Tablet Oral<br />
30 mg+2 mg+<br />
500 mg+ 5.0<br />
mg Tablet Oral<br />
187 ASCORBIC ACID VITAMIN C 100 mg Tablet Oral<br />
188 ASCORBIN VITAMIN C 100 mg Tablet Oral<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
SEARLE DIVISON OF<br />
MONSANTO plc.<br />
(UK) GOKALS LTD 12/31/2011<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 3/1/2012<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 6/1/2011<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 6/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
22<br />
MARCH 1, 2011
189 ASCOREX<br />
190 ASMADRIN<br />
191 ASNAC GEL<br />
192 ASOMEX<br />
193 ASOMEX<br />
BROMHEXINE+GU<br />
AIFENESIN+SALBU<br />
TAMOL Multiple Syrup<br />
EPHEDRINE+PHEN<br />
OBARBITOL+THEO<br />
PHYLLINE Multiple Tablet Oral<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
1.16%w/w+3.<br />
00%w/w+5.0<br />
0%w/w+10.0<br />
0%w/w Gel Topical<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 2.5 mg Tablet Oral<br />
194 ASPARAGINASE ASPARAGINASE 10000 iu/vial<br />
Injectable<br />
Solution<br />
195 ASPIMOL PARACETAMOL 120 mg/5 ml Syrup<br />
196 ASPIRIN<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
Tablet<br />
Dispersible<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
TOBINCO<br />
PHARMACY<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
STARWIN<br />
PRODUCTS LTD.<br />
ALLY PHARMA<br />
OPTIONS PVT.<br />
LTD.<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
STARWIN<br />
PRODUCTS LTD. 10/1/2012<br />
TOBINCO<br />
PHARMACY 6/1/2011<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 8/1/2013<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 8/1/2013<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 11/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
23<br />
MARCH 1, 2011
197 ASPIRIN<br />
198 ASPIRIN<br />
199 ASPIRIN<br />
200 ASPIRIN<br />
201 ASPIRIN DISPERSIBLE<br />
202 ASPITONE<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Dispersible<br />
ACETYLSALICYLIC<br />
ACID 300 mg Tablet Oral<br />
ACETYLSALICYLIC<br />
ACID 300 mg Tablet Oral<br />
ACETYLSALICYLIC<br />
ACID 300 mg Tablet Oral<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
Tablet<br />
Dispersible<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
203 ASTHALEX JUNIOR SALBUTAMOL 2 mg/5 ml Syrup<br />
204 ASTYFER<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
ROCK<br />
CHEMISTS<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 12/31/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
TABLETS (INDIA)<br />
LIMITED<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 11/1/2012<br />
UNICHEM<br />
GHANA LTD. 2/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
24<br />
MARCH 1, 2011
205 ASTYFER LIQUID<br />
206 ASTYMIN FORTE<br />
207 ASTYMIN LIQUID<br />
208 ASTYMIN-C<br />
209 ASUMOD (18yrs +)<br />
210 ASUMOD (7-13yrs)<br />
211 ATACAND<br />
212 ATACAND PLUS<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
AMINO<br />
ACID+MULTIVITAM<br />
INS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AMINO<br />
ACID+MULTIVITAM<br />
INS Multiple Syrup<br />
AMINO<br />
ACID+MULTIVITAM<br />
INS Multiple Oral Drops<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
300 mg + 100<br />
mg<br />
150 mg + 50<br />
mg<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
CANDESARTAN<br />
CILEXETIL 32 mg Tablet Oral<br />
CANDESARTAN+H<br />
YDROCHLOROTHI<br />
AZIDE<br />
16 mg+12.5<br />
mg Tablet Oral<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A SCIENTIFIC<br />
OFFICE<br />
TABLETS (INDIA)<br />
LIMITED<br />
TABLETS (INDIA)<br />
LIMITED<br />
TABLETS (INDIA)<br />
LIMITED<br />
TABLETS (INDIA)<br />
LIMITED<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 4/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 2/1/2013<br />
25<br />
MARCH 1, 2011
213 ATAZOR-100 ATAZANAVIR 100 mg<br />
214 ATAZOR-150 ATAZANAVIR 150 mg<br />
215 ATAZOR-200 ATAZANAVIR 200 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
216 ATENOLOL ATENOLOL 100 mg Tablet Oral<br />
217 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
218 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
219 ATENOLOL ATENOLOL 25 mg Tablet Oral<br />
220 ATENOLOL ATENOLOL 100 mg Tablet Oral<br />
221 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
222 ATENOLOL ATENOLOL 100 mg Tablet Oral<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 12/1/2012<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 12/1/2012<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 12/1/2012<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
26<br />
MARCH 1, 2011
223 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
224 ATENOLOL ATENOLOL 100 mg Tablet Oral<br />
225 ATENOLOL ATENOLOL 100 mg Tablet Oral<br />
226 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
227 ATENOLOL ATENOLOL 25 mg Tablet Oral<br />
228 ATENOLOL ATENOLOL 50 mg Tablet Oral<br />
229 ATENOLOL-DENK ATENOLOL 50 mg Tablet Oral<br />
230 A-TIM TIMOLOL 0.5%w/v Ear Drops<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
AMBICA<br />
PHARMA<br />
SALES<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 8/1/2012<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
SPINTEX<br />
CHEMIST LTD 12/1/2012<br />
27<br />
MARCH 1, 2011
231 ATOCOR<br />
232 ATOSTIN<br />
ATORVASTATIN<br />
CALCIUM 20 mg Tablet Oral<br />
ATORVASTATIN<br />
CALCIUM 20 mg Tablet Oral<br />
233 ATRACADE ATRACURIUM 25 mg<br />
234 ATRACURIUM ATRACURIUM 10 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
235 ATRIZIN CETIRIZINE 10 mg Tablet Oral<br />
236 ATROPINE ATROPINE 1 mg/ml<br />
237 ATROPINE ATROPINE 1 mg/ml<br />
238 ATROPINE ATROPINE 1 mg/ml<br />
239 AUGMENTIN<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
500 mg/100<br />
mg<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Powder<br />
DR REDDY'S DR REDDY'S<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED<br />
(INDIA)<br />
MADRAS<br />
PHARMACEUTICALS<br />
BHARAT SERUMS<br />
AND VACCINES<br />
LTD.<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD HIPLUS,S.A<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
DR. REDDYS<br />
LABORATORIES 12/31/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/21/2015<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED 12/31/2011<br />
VAST FAVOUR<br />
INTERNATIONAL<br />
LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITHKLINE<br />
INTERNATIONAL<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
28<br />
MARCH 1, 2011
240 AUGMENTIN<br />
241 AUGMENTIN<br />
242 AUGPEN<br />
243 AUGPEN<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg/125<br />
mg Tablet Oral<br />
1000 mg/200<br />
mg<br />
Injectable<br />
Powder<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 375 mg Tablet Oral<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
500 mg/125<br />
mg Tablet Oral<br />
244 AVANDIA ROSIGLITAZONE 4 mg Tablet Oral<br />
245 AVASTIN BEVACIZUMAB 25 mg/ml<br />
Injectable<br />
Solution<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
SMITHKLINE<br />
BEECHAM<br />
INT.<br />
GLAXOSMITHKLINE<br />
INTERNATIONAL<br />
GLAXOSMITHKLINE<br />
INTERNATIONAL<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
GLAXO WELLCOME<br />
S.A.(ARANDA,<br />
SPAIN)SB<br />
PHARMCO PUERTO<br />
RICO INC.<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ)<br />
AGGENENTECH<br />
F. HOFFMANN-INC<br />
LA ROCHE (VACAVILLE)GENE<br />
LTD.(Switzerl NTECH INC<br />
and) (OCEANSIDE)<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 5/1/2012<br />
29<br />
MARCH 1, 2011
246<br />
247<br />
AYRTON'S MUSCLE<br />
HEAT RUB<br />
AYRTON'S MUSCLE<br />
HEAT RUB-XTRA<br />
STRONG<br />
248 AZARGA<br />
EUCALYPTUS+MEN<br />
THOL+METHYLSAL<br />
ICYLATE+TURPEN<br />
TINE OIL Multiple<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+TURP<br />
ENTINE<br />
OIL+OTHERS Multiple<br />
BRINZOLAMIDE+TI<br />
MOLOL<br />
249 AZIBEST-250 AZITHROMYCIN 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
10<br />
mg/5ml+5mg<br />
/ml Eye drops<br />
250 AZIGLOBE AZITHROMYCIN 200 mg/5ml<br />
251 AZIGLOBE-500 AZITHROMYCIN 500mg<br />
Tablet film<br />
coated<br />
Oral<br />
Suspension<br />
Tablet film<br />
coated<br />
252 AZILIDE-500 AZITHROMYCIN 500 mg Tablet Oral<br />
253 AZIRON AZITHROMYCIN 250 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
S.A. ALCON-<br />
COUVREUR<br />
N.V.<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
S.A. ALCON-<br />
COUVREUR N.V.<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 10/1/2013<br />
BLUE CROSS<br />
LABORATORIE BLUE CROSS<br />
S<br />
LABORATORIES ROCK CHEMISTS 9/1/2012<br />
GB PHARMA<br />
(uk) LTD.<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MICRO<br />
EXPORTS(LAB<br />
ORATORIES)<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
GB PHARMA<br />
(GH) LTD 2/1/2013<br />
GB PHARMA<br />
(GH) LTD 10/1/2012<br />
J.M. ADDO &<br />
SONS LTD 7/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
30<br />
MARCH 1, 2011
254 AZITHRAL-200 AZITHROMYCIN 40 mg/ml Syrup<br />
255 AZITHRAL-500 AZITHROMYCIN 500 mg Tablet Oral<br />
256 AZOMAX AZITHROMYCIN 500 mg<br />
257 AZOMAX AZITHROMYCIN 200 mg/5ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Powder for<br />
Oral<br />
Suspension<br />
258 AZOPT BRINZOLAMIDE 1% w/v Eye drops<br />
259 BABOO RUB<br />
260 BABY ARALEX<br />
261 BABY-KOFOF<br />
CAPSICUM+METHY<br />
L SALICYLATE<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL<br />
15%w/w/0.5<br />
6%w/w<br />
Ointment<br />
Topical<br />
12.5 mg/120<br />
mg/5 ml Syrup<br />
DILUTE ACETIC<br />
ACID 0.42 ml/5 ml Syrup<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
SANDOZ S.R.L<br />
(ROMANIA)<br />
SANDOZ S.R.L<br />
(ROMANIA)<br />
ALCON-<br />
COUVREUR<br />
BELLS & SONS &<br />
CO LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
DOVE<br />
PHARMACY<br />
LIMITED 12/1/2011<br />
DOVE<br />
PHARMACY<br />
LIMITED 12/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 12/31/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 10/8/2011<br />
TOBINCO<br />
PHARMACY 10/1/2011<br />
31<br />
MARCH 1, 2011
262 BACQURE<br />
CISPLATIN+IMIPEN<br />
EM 500 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
RANBAXY RANBAXY<br />
LABORATORIE LABORATORIES<br />
S LTD. LTD.<br />
263 BACTIFLOX CIPROFLOXACIN 500 mg Tablet Oral MEPHA LTD. MEPHA LTD.<br />
264 BACTROBAN MUPIROCIN 2%w/w<br />
265 BASECAM PIROXICAM 20 mg<br />
266 BASECOLD<br />
267 BASECOLD<br />
Ointment<br />
Topical<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL+PHE 30 mg + 2 mg<br />
NYLPROPANOLAMI + 500 mg +<br />
NE<br />
25 mg Tablet Oral<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PHENYLEPHRIN<br />
E+OTHERS Multiple Syrup<br />
268 BASEFENAC 100 DICLOFENAC 100 mg Tablet Oral<br />
269 BASEVITA<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
GLAXO<br />
OPERATIONS UK<br />
LTDGLAXOSMITHK<br />
LINE UK LIMITED<br />
BHARAT<br />
PARENTERALS<br />
LTDRONAK EXIM<br />
PRIVATE LIMITED<br />
MERCURY MERCURY<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
MERCURY MERCURY<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
BHARAT<br />
PARENTERALS<br />
LTDRONAK EXIM<br />
PRIVATE LIMITED<br />
STABICOAT<br />
PHARMACHEM PVT<br />
LTD<br />
GB PHARMA<br />
(GH) LTD 11/1/2012<br />
PHARMA INFO<br />
CONSULT 2/1/2013<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
BASE PHARMACY<br />
LIMITED 7/1/2012<br />
BASE PHARMACY<br />
LIMITED 2/1/2013<br />
BASE PHARMACY<br />
LIMITED 3/1/2013<br />
BASE PHARMACY<br />
LIMITED 7/1/2012<br />
BASE PHARMACY<br />
LIMITED 7/1/2012<br />
32<br />
MARCH 1, 2011
270 B'CO TABLETS<br />
271 B-COMPLEX STRONG<br />
272 BECLASONE N<br />
273 BECOACTIN<br />
NICOTINAMIDE+RI<br />
BOFLAVIN+THIAMI<br />
NE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
15 mg+1<br />
mg+1 mg Tablet Oral<br />
VITAMIN B<br />
COMPLEX Multiple Tablet Oral<br />
BECLOMETHASON<br />
E+NEOMYCIN<br />
0.025%<br />
w/w/0.5<br />
%w/w<br />
Cream<br />
Topical<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
274 BECOZEE SYRUP MULTIVITAMIN Multiple Syrup<br />
275 BELLA COUGH<br />
276 BELL'S ANTACID MIST.<br />
AMMONIUM<br />
CHLORIDE+CHLOR<br />
PHENIRAMINE+DIP<br />
HENHYDRAMINE+S<br />
ODIUM CI Multiple Syrup<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE Multiple<br />
277 BELL'S BABY COUGH SQUILL OXYMEL<br />
278<br />
BELL'S BABY GRIPE<br />
WATER<br />
Oral<br />
Suspension<br />
0.25 mls/5<br />
mls Syrup<br />
DILL OIL+SODIUM<br />
BICARBONATE Multiple Oral Solution<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
MEYER<br />
ORGANICS<br />
LTD.<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 4/1/2013<br />
ATLAS<br />
PHARMACY<br />
LIMITED 12/1/2011<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
MEYER ORGANICS<br />
LTD.<br />
GEO MEDICORE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
GR PHARMA<br />
LTD. 10/1/2012<br />
GEO MEDICORE<br />
LIMITED 8/1/2013<br />
SAMARA<br />
PHARMACY LTD 3/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
33<br />
MARCH 1, 2011
279<br />
BELL'S BENZYL<br />
BENZOATE<br />
BENZYL<br />
BENZOATE 25%w/v<br />
280 BELL'S CALAMINE CALAMINE BP<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
Lotion<br />
Topical<br />
Lotion<br />
281 BELLS CASTOR OIL CASTOR OIL BP Oral Solution<br />
282<br />
BELL'S CHILDREN<br />
COUGH<br />
CETYLPYRIDINIUM<br />
+IPECACUANHA+O<br />
THERS Multiple Syrup<br />
283 BELL'S EPHEDRINE EPHEDRINE 0.5%w/v Nasal Drops<br />
284 BELL'S EPHEDRINE EPHEDRINE 1%w/v Nasal Drops<br />
285<br />
286<br />
287<br />
BELL'S HYDROGEN<br />
PEROXIDE<br />
BELLS IODINE<br />
TINCTURE IODINE<br />
HYDROGEN<br />
PEROXIDE 6% w/w<br />
BELL'S LIQUID<br />
PARAFFIN PARAFFIN BP.<br />
288 BELL'S MUSCLE RUB<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE Multiple<br />
Topical<br />
solution<br />
Topical<br />
solution<br />
Oral<br />
Emulsion<br />
Ointment<br />
Topical<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
34<br />
MARCH 1, 2011
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
289 BELL'S OLIVE OIL OLIVE OIL BP Oral Solution<br />
290<br />
291<br />
BELL'S POTASSIUM<br />
NITRATE<br />
BELL'S POTASSIUM<br />
PERMANGANATE<br />
292 BELL'S TONIC<br />
POTASSIUM<br />
NITRATE<br />
POTASSIUM<br />
PERMANGANATE<br />
Powder for<br />
Topical<br />
Solution<br />
Powder for<br />
Topical<br />
Solution<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup<br />
293 BELL'S VITAMIN MULTIVITAMIN Multiple Syrup<br />
294 BELL'S VITAMIN C VITAMIN C 50 mg/5ml Syrup<br />
295 BENAGIN DIPYRON 2.5 g/5 ml<br />
296 BENDEX ALBENDAZOLE 200 mg/5 ml<br />
Injectable<br />
Solution<br />
Oral<br />
Suspension<br />
297 BENDROFLUAZIDE BENDROFLUAZIDE 5 mg Tablet Oral<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
GUANDONG<br />
MEDICINES &<br />
HEALTH<br />
PRODUCTS<br />
I/E CORP<br />
CIPLA<br />
LIMITED<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
SISHUI XIERKANG<br />
PHARMACEUTICAL<br />
COMPANY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
BEIJING HOLLEY-<br />
COTEC<br />
PHARMACEUTIC<br />
ALS CO. LTD. 11/1/2011<br />
HEALTHCARE<br />
SERVICES LTD 2/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
35<br />
MARCH 1, 2011
298 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
299 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
300 BENDROFLUAZIDE BENDROFLUAZIDE 5 mg Tablet Oral<br />
301 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
302 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
303 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
304 BENDROFLUAZIDE BENDROFLUAZIDE 5 mg Tablet Oral<br />
305 BENDROFLUAZIDE BENDROFLUAZIDE 2.5 mg Tablet Oral<br />
306 BENDROFLUAZIDE BENDROFLUAZIDE 5 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
36<br />
MARCH 1, 2011
307 BENDROFLUAZIDE BENDROFLUAZIDE 5 mg Tablet Oral<br />
308 BENYLIN CHESTY<br />
309<br />
BENYLIN DMD<br />
DECONGESTANT<br />
310 BENYLIN DRY<br />
311<br />
BENYLIN FOUR FLU<br />
LIQUID<br />
312 BENYLIN ORIGINAL<br />
313 BENYLIN PAEDIATRIC<br />
314<br />
BENZATHINE<br />
PENICILLIN<br />
BROMHEXINE+ORC<br />
IPRENALINE<br />
DEXTROMETHORP<br />
HAN+PSEUDOEPH<br />
EDRINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
5 mg/ 4<br />
mg/5 ml Syrup<br />
15 mg/ 30<br />
mg/5 ml Syrup<br />
DEXTROMETHORP<br />
HAN 15 mg/5 ml Syrup<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL+<br />
PSEUDOEPHEDRIN<br />
E Multiple Syrup<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE<br />
12.50<br />
mg/125 mg/5<br />
mls Syrup<br />
DIPHENHYDRAMIN<br />
E 7 mg/5 ml Syrup<br />
BENZYLPENICILLI<br />
N 1.2 mega<br />
Injectable<br />
Powder<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
ERNEST CHEMISTS<br />
LIMITED<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 8/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 8/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 10/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 7/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
37<br />
MARCH 1, 2011
315<br />
316<br />
BENZATHINE<br />
PENICILLIN<br />
BENZATHINE<br />
PENICILLIN<br />
BENZYLPENICILLI<br />
N 2.4 Mega<br />
BENZYLPENICILLI<br />
N 1.2 mega<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
317 BENZEHOL BENZHEXOL 2 mg Tablet Oral<br />
318 BENZEHOL BENZHEXOL 5 mg Tablet Oral<br />
319 BENZIL ALBENDAZOLE 100 mg/5 mls<br />
Oral<br />
Suspension<br />
320 BENZTROPINE BENZTROPINE 2 mg Tablet Oral<br />
321 BENZTROPINE BENZTROPINE 1 mg/2 ml<br />
322 BENZYL BENZOATE<br />
323 BENZYL PENICILLIN<br />
324 BENZYL PENICILLIN<br />
BENZYL<br />
BENZOATE<br />
BENZYLPENICILLI<br />
N 1 Mega<br />
BENZYLPENICILLI<br />
N 1 Mega<br />
Injectable<br />
Solution<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
ROCK<br />
CHEMISTS<br />
ROCK<br />
CHEMISTS<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
DOVE<br />
PHARMACY<br />
LIMITED 3/1/2013<br />
DOVE<br />
PHARMACY<br />
LIMITED 2/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 2/1/2013<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
ALEMBIC<br />
LIMITED<br />
SINOCHEM JIANGSU<br />
IMPORT &<br />
EXPORTALEMBIC<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
DOVE<br />
PHARMACY<br />
LIMITED 6/1/2013<br />
38<br />
MARCH 1, 2011
325<br />
BENZYLPENICILLIN<br />
SODIUM<br />
326 BETACLAV<br />
BENZYLPENICILLI<br />
N 1mu<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 625 mg Tablet Oral<br />
327 BETAGAN LEVOBUNOLOL 5mg/ml Eye drops<br />
328 BETAPYN<br />
329 BETASOL USP<br />
330 BETNOVATE-C<br />
331 BETOPTIC<br />
CAFFEINE+CODEIN<br />
E+DOXYLAMINE+P<br />
ARACETAMOL<br />
CLOBETASOL<br />
PROPIONATE 0.05% w/v<br />
BETAMETHASONE<br />
+CLIOQUINOL<br />
50<br />
mg+10mg+5<br />
mg+450 mg Tablet Oral<br />
0.10%w/w/3<br />
%w/w<br />
Topical<br />
Lotion<br />
Cream<br />
Topical<br />
BETAXOLOL<br />
HYDROCHLORIDE 5 mg/ml Eye drops<br />
332 BEXFLOXIN CIPROFLOXACIN 500 mg Tablet Oral<br />
SINOCHEM<br />
JIANGSU<br />
IMPORT &<br />
EXPORT<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT.<br />
LTD.<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
LUNAN BETTER<br />
PHARMACEUTICALS<br />
CO. LTD<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2013<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2013<br />
ALLERGAN<br />
PHARMACEUTICALS BERNSWETT<br />
COMPANY LTD 6/1/2011<br />
ADCOCK INGRAM<br />
LIMITED<br />
SHALINA<br />
LABORATORIE GOPALDAS VISRAM<br />
S PVT. LTD. & CO. LTD<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
ALCON-<br />
COUVREUR<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
ALCON-<br />
COUVREUR<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 9/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 10/1/2013<br />
HILLS<br />
PHARMACY 11/1/2011<br />
39<br />
MARCH 1, 2011
333 BEXYMICIN AZITHROMYCIN 500 mg<br />
334 BEXYMICIN-250 AZITHROMYCIN 250 mg<br />
335 BG-MET METFORMIN 500 mg<br />
336 BICOSONS MULTIVITAMIN Multiple Syrup<br />
337 BIFILAC<br />
338 BIFILAC<br />
339 BINA<br />
BACILLUS+CLOSTR<br />
IDIUM+LACTIC<br />
ACID+STREPTOCO<br />
CCUS Multiples<br />
BACILLUS+CLOSTR<br />
IDIUM+LACTIC<br />
ACID+STREPTOCO<br />
CCUS 0.5 g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED<br />
HILLS<br />
PHARMACY 12/31/2011<br />
HILLS<br />
PHARMACY 5/1/2011<br />
Tablet film<br />
coated GVS LABS GVS LABS GVS LABS 8/1/2011<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L 120 mg+2 mg Syrup<br />
340 BIO-E VITAMIN E 400 iu<br />
Powder for<br />
Oral Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
BODYCOTE<br />
PHARMACY<br />
LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
TABLETS (INDIA)<br />
LIMITED<br />
TABLETS (INDIA)<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
OSON'S CHEMIST<br />
LTD 6/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
BODYCOTE<br />
PHARMACY<br />
LIMITED 10/1/2011<br />
DR REDDY'S BANNER<br />
LABORATORIE PHARMACAPS<br />
S LIMITED (INDIA) PVT LTD GOKALS LTD 10/1/2012<br />
40<br />
MARCH 1, 2011
341 BIOVID FORTE<br />
342 BIOZIVIT<br />
VITAMIN<br />
B1+VITAMIN<br />
B6+VITAMIN B12<br />
242.5 mg +<br />
250 mg + 1<br />
mg<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup<br />
343 BISACODYL BISACODYL 10 mg Suppository<br />
344 BISOPROLOL BISOPROLOL 5 mg Tablet Oral<br />
345 BISOPROLOL BISOPROLOL 10 mg Tablet Oral<br />
346 BISOPROLOL BISOPROLOL 10 mg Tablet Oral<br />
347 BIVACYN AEROSOL<br />
348 BIVEK<br />
BACITRACIN+NEO<br />
MYCIN<br />
LAMIVUDINE+ZIDO<br />
VUDINE<br />
165,000<br />
iu/12,500<br />
iu/150 mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated HOVID Bhd. HOVID Bhd.<br />
Powder for<br />
Topical<br />
Solution<br />
300 mg/150<br />
mg Caplets oral<br />
SENES<br />
PHARMA CO.<br />
LTD<br />
PREGEN EXPORTS<br />
(INDIA)<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
BEDITA<br />
PHARMACY 2/1/2013<br />
SENES PHARMA<br />
CO. LTD 3/6/2050<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
PALB<br />
PHARMACEUTIC<br />
ALS 9/1/2012<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 5/1/2012<br />
41<br />
MARCH 1, 2011
349 BODIPLEX<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
350 BONVIVA IBANDRONIC ACID 150 mg Tablet Oral<br />
351 BONVIVA IBANDRONIC ACID 1 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
352 BORIC SPIRIT BORIC ACID 2% Ear Drops<br />
353 BRINERDIN<br />
354 BRIVITY<br />
CLOPAMIDE+DIHY<br />
DROERGOCRISTIN<br />
E+RESERPINE<br />
VITAMIN<br />
B1+VITAMIN<br />
B6+VITAMIN B12<br />
0.5 mg/5<br />
mg/0.10 mg Tablet Oral<br />
200 mg+50<br />
mg+1000mcg Tablet Oral<br />
355 BRONCHOLIN SALBUTAMOL 2 mg/5ml Syrup<br />
356 BRONCHOLIN SALBUTAMOL 4 mg Tablet Oral<br />
J.M. ADDO &<br />
SONS LTD<br />
F. HOFFMAN<br />
LA-ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
NOVARTIS<br />
PHARMA<br />
GmbH<br />
CENTAUR<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
F. HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
F. HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
KAMA INDUSTRIES<br />
LTD.<br />
NOVARTIS FARMA<br />
SPA (ITALY)<br />
CENTAUR<br />
PHARMACEUTICALS<br />
PVT LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
J.M. ADDO &<br />
SONS LTD 12/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 10/10/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
SUPRA PHARMA<br />
LIMITED 11/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
42<br />
MARCH 1, 2011
357 BRONCHOPED<br />
358 BRUCAPS<br />
359 BUPICAN HEAVY<br />
360 BUSOMIN<br />
361 CA-C<br />
362 CACHCLAV<br />
363 CACHCLAV<br />
364 CACHLAV<br />
TERBUTALINE<br />
SULPHATE +<br />
AMMONIUM<br />
CHLORIDE +<br />
SODIUM CITRATE<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
1.25mg +<br />
60mg + 25mg Syrup<br />
325 mg/200<br />
mg/30 mg<br />
BUPIVACAINE+GL<br />
UCOSE 0.5% w/v<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
HYOSCINE<br />
BUTYLBROMIDE 10 mg Tablet Oral<br />
ASCORBIC<br />
ACID+CALCIUM 1000 mg<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
875mg + 125<br />
mg<br />
500 mg + 125<br />
mg<br />
200mg +<br />
28.5mg<br />
Tablet<br />
Dispersible<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Powder for<br />
Oral<br />
Suspension<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
NOVARTIS<br />
PHARMA AG<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
PHARMA-<br />
Q(Pty)Ltd.<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
SUPRA PHARMA<br />
LIMITED 6/1/2012<br />
ESKAY<br />
THERAPEUTICS 11/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
DAAMASS CO.<br />
LIMITED 11/1/2012<br />
DAAMASS CO.<br />
LIMITED 11/1/2012<br />
DAAMASS CO.<br />
LIMITED 12/1/2012<br />
43<br />
MARCH 1, 2011
365 CACHLAV<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
366 CACHTAX CEFOTAXIME 1 g<br />
125mg +<br />
31.25mg<br />
367 CACHTAX CEFOTAXIME 500 mg<br />
368 CADUET<br />
369 CADUET<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
AMLODIPINE+ART<br />
OVASTATIN 5 mg/10 mg Tablet Oral<br />
AMLODIPINE+ART<br />
OVASTATIN 10 mg/10 mg Tablet Oral<br />
370 CAELYX DOXORUBICIN 2 mg/ml<br />
371 CAFALGIN<br />
372 CAFALGIN JUNIOR<br />
Injectable<br />
Solution<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL Multiple Tablet Oral<br />
DIPHENHYDRAMIN<br />
E+IBUPROFEN+PA<br />
RACETAMOL<br />
12.5<br />
mg/+120<br />
mg/5 mls Syrup<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
PFIZER IRELAND<br />
PHARMACEUTICALS<br />
PFIZER IRELAND<br />
PHARMACEUTICALS<br />
DAAMASS CO.<br />
LIMITED 12/1/2012<br />
DAAMASS CO.<br />
LIMITED 6/1/2012<br />
DAAMASS CO.<br />
LIMITED 6/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 12/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 7/1/2013<br />
BEN VENUE<br />
LABORATORIES<br />
INC GOKALS LTD 3/1/2012<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/13/2011<br />
44<br />
MARCH 1, 2011
373 CAFERGOT<br />
374 CALAMINE<br />
375<br />
CALAMINE OINTMENT<br />
BPC<br />
376 CALCEVIT<br />
ERGOTAMINE+CAF<br />
FEINE 1 mg/100 mg Tablet Oral<br />
CALAMINE+ZINC<br />
OXIDE<br />
CALCIUM+VITAMIN<br />
D<br />
15%w/v +<br />
5%w/v<br />
377 CALCIGARD RETARD NIFEDIPINE 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
Lotion<br />
500 mg/250<br />
IU Tablet Oral<br />
NOVARTIS<br />
PHARMA AG<br />
SM<br />
PHARMACEUT<br />
ICALS SDN.<br />
BHD.<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD (UK)<br />
SM<br />
PHARMACEUTICALS<br />
SDN. BHD.<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
RENIE CHEMISTS<br />
LIMITED 8/1/2013<br />
Topical<br />
Suspension DANNEX LTD DANNEX LTD DANNEX LTD 4/1/2013<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
378 CALCINOL ATENOLOL 50 mg Tablet Oral<br />
379 CALCINOL ATENOLOL 100 mg Tablet Oral<br />
380 CALCIRON-Z<br />
381 CALCIUM GLUCONATE<br />
382 CALCIUM GLUCONATE<br />
CALCITROL+CALCI<br />
UM<br />
CARBONATE+ZINC<br />
SUPHATE<br />
0.25 mcg +<br />
500 mg + 7.5<br />
mg<br />
CALCIUM<br />
GLUCONATE 10%w/v<br />
CALCIUM<br />
GLUCONATE 10%w/v<br />
Capsule oral<br />
(Softgel)<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
BAFNA<br />
PHARMACEUT<br />
ICALS LTD<br />
BAFNA<br />
PHARMACEUT<br />
ICALS LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
SOCOMEX<br />
PHARMACY LTD. 12/16/2012<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 2/1/2013<br />
BAFNA<br />
PHARMACEUTICALS<br />
LTD<br />
BAFNA<br />
PHARMACEUTICALS<br />
LTD<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 5/1/2013<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 5/1/2013<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 3/1/2012<br />
45<br />
MARCH 1, 2011
383 CALCIUM-SANDOZ<br />
384 CALFLAVONE<br />
385<br />
386<br />
387<br />
CAMOQUIN-PLUS<br />
(PAEDIATRIC)<br />
CAMOQUIN-PLUS<br />
ADULT<br />
CAMOSUNATE<br />
PAEDIATRIC<br />
388 CAMOSUNATE PDR<br />
CALCIUM<br />
CARBONATE 500 mg<br />
CALCIUM+VITAMIN<br />
D+IPRIFLAVONE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Dispersible<br />
1000 mg/200<br />
iu/300 mg Tablet Oral<br />
160 mg/50<br />
mg/5 mls<br />
AMODIAQUINE+AR<br />
TESUNATE 150/50mg<br />
AMODIAQUINE+AR<br />
TESUNATE 75/25mg<br />
389 CANALEX CLOTRIMAZOLE 1%w/w<br />
Oral<br />
Suspension<br />
100 mg/300<br />
mg Tablet Oral<br />
Powder for<br />
Oral<br />
Suspension<br />
Powder for<br />
Oral<br />
Suspension<br />
Cream<br />
Topical<br />
NOVARTIS<br />
PHARMA AG<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
TABLETS (INDIA)<br />
LIMITED<br />
ADAMS<br />
PHARMACEUTICALS<br />
GROUP CO.<br />
LTDPFIZER<br />
AFRIQUE, DAKAR<br />
SENEGAL<br />
ADAMS<br />
PHARMACEUTICALS<br />
GROUP CO. LTD<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/5/2012<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 11/1/2011<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 7/1/2011<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 7/1/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 9/1/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 9/1/2011<br />
UNICHEM<br />
GHANA LTD. 12/1/2012<br />
46<br />
MARCH 1, 2011
390 CANDID CLOTRIMAZOLE 1%w/v<br />
391 CANDID B<br />
392<br />
393<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
CANDID<br />
CLOTRIMAZOLE CLOTRIMAZOLE 1% w/v<br />
CANDID DUSTING<br />
POWDER CLOTRIMAZOLE 1%w/v<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
1%<br />
w/v/0.25%<br />
w/w Gel Topical<br />
Topical<br />
Lotion<br />
Powder for<br />
topical use<br />
394 CANDID MOUTH PAINT CLOTRIMAZOLE 1%w/v Gel Oral<br />
395 CANDID V CLOTRIMAZOLE 2%w/v Gel Topical<br />
396 CANDID V1 CLOTRIMAZOLE 500 mg<br />
397 CANDID V6 CLOTRIMAZOLE 100 mg<br />
Tablet<br />
Vaginal<br />
Tablet<br />
Vaginal<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
BERNSWETT<br />
COMPANY LTD 5/1/2012<br />
BERNSWETT<br />
COMPANY LTD 4/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
47<br />
MARCH 1, 2011
398 CANDIDERM<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+GENTAMYCIN Multiple<br />
399 CANEM MEROPENEM 500 mg<br />
400 CANEM MEROPENEM 1 g<br />
401 CAPAC<br />
402 CAPSITOP<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
250 mg/30<br />
mg/125 mg Tablet Oral<br />
1.16%w/v/0.<br />
025<br />
%w/v/1.0%w<br />
/w/3%w/w Gel Topical<br />
403 CAPTORPIL CAPTOPRIL 25 mg Tablet Oral<br />
404 CAPTORPIL CAPTOPRIL 25 mg Tablet Oral<br />
405 CAPTORPIL CAPTOPRIL 12.5 mg Tablet Oral<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CACHET<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
ALKEM<br />
LABORATORIES<br />
LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
STEDMAN<br />
PHARMACEUTICALS<br />
PVT. LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 3/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
48<br />
MARCH 1, 2011
406 CAPTORPIL CAPTOPRIL 25 mg Tablet Oral<br />
407 CAPTORPIL DENK CAPTOPRIL 25 mg Tablet Oral<br />
408 CARBAMAZEPINE CARBAMAZEPINE 200 mg Tablet Oral<br />
409 CARBAMAZEPINE CARBAMAZEPINE 200 mg Tablet Oral<br />
410 CARBOCISTEINE CARBOCISTEINE 2%w/v Syrup<br />
411 CARDILAT-20 NIFEDIPINE 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Extended<br />
Realese<br />
412 CARDITAS RETARD NIFEDIPINE 20 mg Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROCK<br />
CHEMISTS<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
BELCO<br />
PHARMA PVT<br />
LTD<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
G. D. COOPER &<br />
CO. LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
G. D. COOPER &<br />
CO.<br />
LIMITEDERNEST<br />
CHEMISTS LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
G. D. COOPER &<br />
CO.<br />
LIMITEDERNEST<br />
CHEMISTS LIMITED ROCK CHEMISTS 2/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
BELCO PHARMA<br />
PVT LTD<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD -DEHRADUN<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2012<br />
HILLS<br />
PHARMACY 2/1/2012<br />
GR PHARMA<br />
LTD. 10/1/2011<br />
49<br />
MARCH 1, 2011
413 CARDOVASC XL NIFEDIPINE 30 mg<br />
414<br />
CARTISAFE FORTE<br />
MSM<br />
CALCIUM+GLUCOS<br />
AMINE+METHYL<br />
SULPHONYL<br />
METHANE+VITAMI<br />
N D3<br />
750 mg/624<br />
mg/1.507<br />
mg/200 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet film<br />
coated<br />
415 CARVEDILOL CARVEDILOL 25 mg Tablet Oral<br />
416 CARVEDILOL CARVEDILOL 12.5 mg Tablet Oral<br />
417 CARVO<br />
418 CASPAGIN<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL<br />
CAFFEINE+PARAC<br />
ETAMOL<br />
500 mg/25<br />
mg Caplets oral<br />
500 mg/30<br />
mg Tablet Oral<br />
419 CATAFLAM DICLOFENAC 1.50%w/v Oral Drops<br />
420 CATAFLAM DICLOFENAC 50 mg Tablet Oral<br />
HILLS<br />
PHARMACY<br />
JENBURKT<br />
PHARMACEUT<br />
ICALS LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
BELCO PHARMA<br />
PVT LTD<br />
JENBURKT<br />
PHARMACEUTICALS<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
HILLS<br />
PHARMACY 9/1/2012<br />
ZETA<br />
PHARMACY LTD 7/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
SAMARA<br />
PHARMACY LTD 3/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 2/1/2012<br />
50<br />
MARCH 1, 2011
421 CEBROTONIN PIRACETAM 800 mg Tablet Oral<br />
422 CEFALEX CEPHALEXIN 125 mg/5ml<br />
423 CEFALEX CEPHALEXIN 250 mg<br />
424 CEFATAX CEFOTAXIME 1 g<br />
425 CEFOGRAM CEFTRIAXONE 1000 mg<br />
426 CEFORON-1000 CEFOTAXIME 1000 mg<br />
427 CEFORON-500 CEFOTAXIME 500 mg<br />
428 CEFUKEN CEFUROXIME 125 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Powder for<br />
Oral<br />
Suspension<br />
429 CEFUNAT CEFUROXIME 250 mg Tablet Oral<br />
430 CEFUNAT CEFUROXIME 125 mg/5ml<br />
Powder for<br />
Oral<br />
Suspension<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
ORCHID<br />
HEALTHCARE<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
INDUSKEN<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
ORCHID<br />
HEALTHCARE<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
WEST COAST<br />
PHARMACEUTICAL<br />
WORKS LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
UNICHEM<br />
GHANA LTD. 3/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 5/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 10/1/2012<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
KINAPHARMA<br />
LTD 9/1/2011<br />
51<br />
MARCH 1, 2011
431 CEFUNAT-500 CEFUROXIME 500 mg Tablet Oral<br />
432 CEFURO-DOR CEFUROXIME 250 mg Tablet Oral<br />
433 CEFURO-DOR CEFUROXIME 750 mg<br />
434 CEFUROX CEFUROXIME 750 mg/vial<br />
435 CELEMIN TM AMINO ACIDS 10% w/v<br />
436 CELEPID FAT EMULSION 10% w/v<br />
437 CEROXIM CEFUROXIME 500 mg<br />
438 CEROXIM CEFUROXIME 250 mg<br />
439 CETA-IBU<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Solution<br />
Injectable<br />
Suspension<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
100 mg + 125<br />
mg/5mls Syrup<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
SHANGDONG<br />
REYOUNG<br />
PHARMACEUTICALS<br />
CO. LTD<br />
OKASA PHARMA<br />
PVT LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
RANBAXY RANBAXY<br />
LABORATORIE LABORATORIES<br />
S LTD. LTD.<br />
RANBAXY RANBAXY<br />
LABORATORIE LABORATORIES<br />
S LTD. LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
MISSION VIVACARE<br />
LIMITED<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 6/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 6/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 11/1/2012<br />
SUPRA PHARMA<br />
LIMITED 10/1/2012<br />
GB PHARMA<br />
(GH) LTD 12/31/2012<br />
GB PHARMA<br />
(GH) LTD 12/31/2012<br />
GB PHARMA<br />
(GH) LTD 10/1/2011<br />
52<br />
MARCH 1, 2011
440 CETAPOL PARACETAMOL 120mg/5 ml Syrup<br />
441 CETAPOL PM<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
12.5 ml + 120<br />
mg /5 ml Syrup<br />
442 CETIL 500 CEFUROXIME 500 mg Tablet Oral<br />
443 CETRIM ANTISEPTIC<br />
CETRIMIDE+VITAM<br />
IN E Multiple Solid Soap<br />
444 CEVITE VITAMIN C 500 mg Tablet Oral<br />
445 C-FLOX CIPROFLOXACIN 500 mg Tablet Oral<br />
446 CHILDCARE ASCORBIN VITAMIN C 100 mg/5 mls Syrup<br />
447<br />
CHILDCARE NIGHT<br />
REST<br />
448 CHLO i<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE Multiple<br />
Oral<br />
Suspension<br />
CHLORAMPHENIC<br />
OL 5%w/v Ear Drops<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
LUPIN<br />
LIMITED LUPIN LIMITED<br />
PSYCHOTROP<br />
IC INDIA LTD<br />
SHALINA<br />
LABORATORIE<br />
S PVT. LTD.<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
TOBINCO<br />
PHARMACY<br />
PSYCHOTROPIC<br />
INDIA LTD<br />
FREDUN<br />
PHARMACEUTICALS<br />
LTDSHALINA<br />
LABORATORIES<br />
PVT. LTD.<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
SHREECHEM<br />
LABORATORIES<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 8/1/2012<br />
RYZENPHARMA<br />
LIMITED 4/1/2012<br />
J.M. ADDO &<br />
SONS LTD 9/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 9/1/2011<br />
GR PHARMA<br />
LTD. 7/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
53<br />
MARCH 1, 2011
449 CHLO i<br />
450 CHLOi<br />
451 CHLORAMPENICOL<br />
452 CHLORAMPENICOL<br />
453 CHLORAMPENICOL<br />
454 CHLORAMPENICOL<br />
455 CHLORAMPENICOL<br />
456 CHLORAMPHENICOL<br />
457 CHLORAMPHENICOL<br />
458 CHLORAMPHENICOL<br />
459 CHLORAMPHENICOL<br />
CHLORAMPHENIC<br />
OL 0.5%w/v Eye drops<br />
CHLORAMPHENIC<br />
OL 0.5% w/v Eye drops<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
TOBINCO<br />
PHARMACY<br />
TOBINCO<br />
PHARMACY<br />
CHLORAMPHENIC<br />
TROGE<br />
MEDICAL<br />
OL 1% w/v Eye ointment GMBH<br />
CHLORAMPHENIC<br />
OL 0.5% Eye drops<br />
CHLORAMPHENIC<br />
OL 250 mg<br />
CHLORAMPHENIC<br />
OL 1 g<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
CHLORAMPHENIC<br />
OL 5% w/v Ear Drops<br />
CHLORAMPHENIC<br />
OL 1 g<br />
Injectable<br />
Powder<br />
CHLORAMPHENIC<br />
OL 0.50 %w/v Eye drops<br />
CHLORAMPHENIC<br />
OL 0.5% w/v Eye drops<br />
CHLORAMPHENIC<br />
OL 250 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
SHREECHEM<br />
LABORATORIES<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
SHREECURE<br />
PHARMACEUTICALS<br />
PVT LTD<br />
ADORE<br />
PHARMACEUTICALS<br />
PVT. LTD.<br />
ADORE<br />
PHARMACEUTICALS<br />
PVT. LTD.<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
TROGE MEDICAL<br />
GMBH<br />
TROGE MEDICAL<br />
GMBH<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
TOBINCO<br />
PHARMACY 10/1/2012<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
UNICHEM<br />
GHANA LTD. 7/1/2011<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
54<br />
MARCH 1, 2011
460 CHLORAMPHENICOL<br />
461 CHLORHEXIDINE CHLORHEXIDINE<br />
CHLORAMPHENIC<br />
Oral<br />
OL 125 mg/5 mls Suspension<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
solution<br />
462 CHLOROQUINE CHLOROQUINE 250 mg Tablet Oral<br />
463 CHLORPHENIRAMINE<br />
464<br />
465<br />
466<br />
467<br />
CHLORPHENIRAMI<br />
NE 4 mg Tablet Oral<br />
CHLORPROMAZINE<br />
HYDROCHLORIDE CHLORPROMAZINE 50 mg Tablet Oral<br />
CHLORPROMAZINE<br />
HYDROCHLORIDE CHLORPROMAZINE 100 mg Tablet Oral<br />
CHLORPROMAZINE<br />
HYDROCHLORIDE CHLORPROMAZINE 25 mg/ml<br />
CHLORPROMAZINE<br />
HYDROCHLORIDE CHLORPROMAZINE 25 mg/ml<br />
468 CHOCOFER FOLIC ACID+IRON<br />
469 CHYROMAL<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
50 mg/0.5<br />
mg/5 ml Syrup<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD MEDPHARMA<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
PHARMANOV<br />
A LTD.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
CHYMOTRYPSIN+T<br />
RYPSIN 100, 000 IU Tablet Oral COOPER S.A.<br />
470 CIFLOX CIPROFLOXACIN 500 mg Tablet Oral<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
PHARMANOVA<br />
LTD. 2/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
GALPHA GALPHA<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
FAMAR S.A<br />
(GREECE)<br />
ERNEST CHEMISTS<br />
LIMITED<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 3/1/2013<br />
KENDICKS<br />
PHARMACY 6/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 11/1/2011<br />
55<br />
MARCH 1, 2011
471 CIKLAVIT<br />
ASCORBIC<br />
ACID+PROTEIN+ZI<br />
NC Multiple Syrup<br />
472 CILOXAN CIPROFLOXACIN 3 mg Eye drops<br />
473 CIMETIDINE CIMETIDINE 400 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
474 CIPLOX CIPROFLOXACIN 0.3% w/v Eye ointment<br />
475 CIPLOX CIPROFLOXACIN<br />
200 mg/100<br />
mls<br />
476 CIPLOX CIPROFLOXACIN 0.3% w/v<br />
Injectable<br />
Solution<br />
Ear/Eye<br />
Drops<br />
477 CIPRINOL CIPROFLOXACIN 500 mg Tablet Oral<br />
478 CIPRINOL I.V. CIPROFLOXACIN 2 mg/ml<br />
Injectable<br />
Solution<br />
NEIMETH<br />
INT'L PHARM.<br />
PLC<br />
ALCON-<br />
COUVREUR<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
NEIMETH INT'L<br />
PHARM. PLC<br />
ALCON-<br />
COUVREUR<br />
G. D. COOPER &<br />
CO. LIMITED<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 3/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 12/31/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 2/1/2013<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 12/31/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
STARWIN<br />
PRODUCTS LTD. 7/1/2011<br />
ATLAS<br />
PHARMACY<br />
LIMITED 2/1/2012<br />
ATLAS<br />
PHARMACY<br />
LIMITED 3/1/2013<br />
56<br />
MARCH 1, 2011
479 CIPROBAY CIPROFLOXACIN 750 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
480 CIPROBIOTIC-FORTE CIPROFLOXACIN 500 mg Tablet Oral<br />
481 CIPROCIR CIPROFLOXACIN 0.3 % w/v<br />
Ear/Eye<br />
Drops<br />
482 CIPRO-DOR CIPROFLOXACIN 500.00 mg Tablet Oral<br />
483 CIPROFLOXACIN CIPROFLOXACIN 500 mg Tablet Oral<br />
484 CIPROFLOXACIN CIPROFLOXACIN 2mg/ml<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
485 CIPROFLOXACIN CIPROFLOXACIN 500 mg Tablet Oral<br />
BAYER -<br />
SCHERING<br />
PHARMA BAYER - SCHERING<br />
AFRICA GMBH PHARMA<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
BEDITA<br />
PHARMACY<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
MICRO<br />
EXPORTS(LAB<br />
ORATORIES)<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2012<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 4/1/2013<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
G. D. COOPER &<br />
CO. LIMITED<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
OSON'S CHEMIST<br />
LTD 7/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 10/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
GR PHARMA<br />
LTD. 12/1/2011<br />
FAR EAST<br />
MERCANTILE 4/1/2012<br />
57<br />
MARCH 1, 2011
486 CIPROFLOXACIN CIPROFLOXACIN 250 mg Tablet Oral<br />
487 CIPROFLOXACIN CIPROFLOXACIN 500 mg Tablet Oral<br />
488 CIPROKAM CIPROFLOXACIN 500 mg Tablet Oral<br />
489 CIPROKIN CIPROFLOXACIN 500 mg Tablet Oral<br />
490 CIPROLAG CIPROFLOXACIN 500 mg Caplets oral<br />
491 CIPROLEM CIPROFLOXACIN 500 mg Tablet Oral<br />
492 CIPROLET CIPROFLOXACIN 2 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
493 CIPROLEX CIPROFLOXACIN 500 mg Tablet Oral<br />
494 CIPROMED CIPROFLOXACIN 500 mg<br />
Tablet for<br />
oral solution<br />
MICRO<br />
EXPORTS(LAB<br />
ORATORIES)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
HANS-E.<br />
LEMBCKE HANS-E. LEMBCKE<br />
DR REDDY'S DR REDDY'S<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
ABYAK<br />
PHARMA LTD.<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
FAR EAST<br />
MERCANTILE 4/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 9/1/2013<br />
FRED'S<br />
PHARMACY 4/1/2011<br />
DR. REDDYS<br />
LABORATORIES 5/1/2013<br />
UNICHEM<br />
GHANA LTD. 2/7/2013<br />
XL LABORATORIES<br />
PVT LTD 3/1/2012<br />
58<br />
MARCH 1, 2011
495 CIPROSAN CIPROFLOXACIN 0.3%w/v<br />
496 CIPROTIN<br />
CIPROFLOXACIN+<br />
TINIDAZOLE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ear/Eye<br />
Drops<br />
500 mg/400<br />
mg Tablet Oral<br />
497 CIPROVEN CIPROFLOXACIN 500 mg Tablet Oral<br />
498 CIPROWIN 500 CIPROFLOXACIN 500 mg Tablet Oral<br />
499 CIPROX CIPROFLOXACIN 0.2%w/v<br />
500 CIPROZID DS CIPROFLOXACIN 500 mg<br />
501 CIROCROM<br />
502 CIROTAMIN<br />
503 CIROTAMIN<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
Tablet film<br />
coated<br />
SODIUM<br />
CROMOGLYCATE 2%w/v Eye drops<br />
AMINO<br />
ACIDS+CYPROHEP<br />
TADINE+MINERALS<br />
+MULTIVITAMIN Multiple Caplets oral<br />
CYPROHEPTADINE<br />
+MULTIVITAMIN+P<br />
ROTEIN Multiple Syrup<br />
SANKA (UK)<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
SRAVANI<br />
LABS LIMITED<br />
SHREECHEM<br />
LABORATORIES PASS PHARMACY 4/1/2012<br />
MADRAS<br />
PHARMACEUTICALS<br />
SRAVANI LABS<br />
LIMITED<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
<strong>DRUG</strong><br />
INTERNATION<br />
AL LIMITED<br />
AMBICA<br />
PHARMA<br />
SALES<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
<strong>DRUG</strong><br />
INTERNATIONAL<br />
LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
D. K. D.<br />
PHARMACY LTD 8/1/2013<br />
DOVE<br />
PHARMACY<br />
LIMITED 12/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
BENNYVEE<br />
CHEMISTS 9/1/2012<br />
SPINTEX<br />
CHEMIST LTD 10/1/2012<br />
OSON'S CHEMIST<br />
LTD 4/8/2015<br />
OSON'S CHEMIST<br />
LTD 2/1/2012<br />
59<br />
MARCH 1, 2011
504 CITRO-SODA ORANGE<br />
CITRIC<br />
ACID+SODIUM<br />
CITRATE+SODIUM<br />
BICARBONATE+TA<br />
RTARIC ACID Multiple<br />
505 CLAFORAN CEFOTAXIME 1 g<br />
506 CLAMOXIN<br />
507 CLAMOXIN<br />
508<br />
CLAMOXIN 457<br />
SUSPENSION<br />
509 CLAMOXIN 625<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
125<br />
mg/31.25<br />
mg/5 mls<br />
250 mg/62.5<br />
mg/5 ml<br />
400 mg+57<br />
mg<br />
510 CLAMYSOL CLINDAMYCIN 150 mg/1 ml<br />
511 CLANOXY<br />
512 CLAVICIN<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
Powder for<br />
Oral<br />
Suspension GVS LABS<br />
Powder for<br />
Oral<br />
Suspension GVS LABS<br />
Oral<br />
Suspension<br />
500 mg+125<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 625 mg Tablet Oral<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
600 mg/100<br />
mg<br />
Injectable<br />
Powder<br />
BLISS GVS<br />
PHARMA LTD<br />
BLISS GVS<br />
PHARMA LTD<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT.<br />
LTD.<br />
ADCOCK INGRAM<br />
HEALTHCARE(PTY)<br />
LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
AVENTIS PHARMA<br />
(PTY) LIMITED GOKALS LTD 8/1/2013<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
BLISS GVS PHARMA<br />
LTD<br />
BLISS GVS PHARMA<br />
LTD<br />
HENAN TOPFOND<br />
PHARMACEUTICALS<br />
GALPHA GALPHA<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 4/24/2013<br />
TOBINCO<br />
PHARMACY 4/1/2011<br />
TOBINCO<br />
PHARMACY 4/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 10/1/2012<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 3/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
60<br />
MARCH 1, 2011
513 CLAVICIN 1.2g<br />
514 CLAVU-DOR 625<br />
515 CLAVULIN<br />
516 CLAVULIN<br />
517 CLEAR INHALER<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 1g + 0.2 g<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
500 mg/125<br />
mg Tablet Oral<br />
500<br />
mg/125mg Tablet Oral<br />
228.5 mg/5<br />
ml<br />
CAMPHOR+EUCAL<br />
YPTUS<br />
OIL+MENTHOL+ME<br />
THYLSALICYLATE Multiple<br />
Powder for<br />
Oral<br />
Suspension<br />
518 CLERMUCOUS CARBOCISTEINE 125 mg/5 mls Syrup<br />
519 CLEXANE 2000 IU ENOXAPARIN<br />
20 mg/ 0.2<br />
ml<br />
520 CLEXANE 40 ENOXAPARIN 40 mg/0.4 ml<br />
Inhalational<br />
Vapour<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SMITHKLINE<br />
BEECHAM<br />
INT.<br />
SMITHKLINE<br />
BEECHAM<br />
INT.<br />
MERCURY<br />
HEALTHCARE<br />
PVT. LTD<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SANOFI<br />
AVENTIS<br />
FRANCE<br />
RHONE-<br />
POULENC<br />
RORER SA<br />
(PTY)<br />
LTD(SOUTH<br />
AFRICA)<br />
STRIDES ARCOLAB<br />
LIMITED<br />
SHANGDONG<br />
REYOUNG<br />
PHARMACEUTICALS<br />
CO. LTD<br />
SMITHKLINE<br />
BEECHAM INT.<br />
SMITHKLINE<br />
BEECHAM INT.<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED<br />
AVENTIS<br />
INTERCONTINENTA<br />
L<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 10/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
ROKMER<br />
PHARMA<br />
LIMITED 9/1/2011<br />
HILLS<br />
PHARMACY 12/31/2011<br />
GOKALS-<br />
LABOREX LTD 9/1/2012<br />
RHODIA<br />
ORGANIQUE FINE<br />
LTD.(UK) GOKALS LTD 9/1/2013<br />
61<br />
MARCH 1, 2011
521 CLEXANE 6000 IU ENOXAPARIN<br />
522 CLEXANE 8000 IU ENOXAPARIN<br />
523 CLODOL EXTRA GEL<br />
60 mg/ 0.6<br />
ml<br />
80 mg/ 0.8<br />
ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE MULTIPLE Gel Oral<br />
524 CLOFENEX-100 DICLOFENAC 100 mg<br />
525 CLOFENEX-50 DICLOFENAC 50 mg<br />
526 CLOMAZOLE CLOTRIMAZOLE 1 %W/W<br />
527 CLOMID<br />
528 CLOMIZOL<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Cream<br />
Topical<br />
CLOMIFENE<br />
CITRATE 50 mg Tablet Oral<br />
CLOTRIMAZOLE+D<br />
EXAMETHASONE+<br />
NEOMYCIN<br />
529 CLOTRI-DENK CLOTRIMAZOLE 1%w/w<br />
Cream<br />
Ophthalmic<br />
Cream<br />
Topical<br />
SANOFI<br />
AVENTIS<br />
FRANCE<br />
SANOFI<br />
AVENTIS<br />
FRANCE<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
ABYAK<br />
PHARMA LTD.<br />
AVENTIS<br />
INTERCONTINENTA<br />
L<br />
AVENTIS<br />
INTERCONTINENTA<br />
L<br />
MISSION VIVACARE<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
XL<br />
LABORATORIE XL LABORATORIES<br />
S PVT LTD PVT LTD<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
MODI-<br />
MUNDIOHARMA<br />
LIMITED<br />
RUBIEPHARM<br />
ARZNEIMITTEL<br />
GmbH<br />
GOKALS-<br />
LABOREX LTD 9/1/2012<br />
GOKALS-<br />
LABOREX LTD 9/1/2012<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
ABYAK PHARMA<br />
LTD. 12/1/2012<br />
SECOM<br />
PHARMACY LTD 12/1/2012<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
BONS<br />
PHARMACY LTD 5/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
62<br />
MARCH 1, 2011
530 CLOTRI-DENK 100 CLOTRIMAZOLE 100 mg<br />
531 CLOTRY CLOTRIMAZOLE 1% w/w<br />
532 CLOXACILLIN CLOXACILLIN 250 mg<br />
533 CLOXACILLIN CLOXACILLIN 125 mg/5 mls<br />
534 CLOXACILLIN CLOXACILLIN 250 mg<br />
535 CO-AMOX<br />
536 COARSUCAM ADULT<br />
537 COARSUCAM CHILD<br />
538 COARSUCAM INFANT<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Vaginal<br />
Cream<br />
Topical<br />
Injectable<br />
Powder<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
875 mg/125<br />
mg Tablet Oral MEPHA LTD. MEPHA LTD.<br />
100 mg/270<br />
mg Tablet Oral<br />
100 mg/270<br />
mg Tablet Oral<br />
25 mg/67.5<br />
mg Tablet Oral<br />
SANOFI-<br />
AVENTIS<br />
(COTE<br />
DIVIORE)<br />
SANOFI-<br />
AVENTIS<br />
(COTE<br />
DIVIORE)<br />
SANOFI-<br />
AVENTIS<br />
(COTE<br />
DIVIORE)<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
OSON'S CHEMIST<br />
LTD 9/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 4/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
PHARMA INFO<br />
CONSULT 3/1/2013<br />
SANOFI-AVENTIS<br />
(MOROCCO) GOKALS LTD 4/14/2012<br />
SANOFI-AVENTIS<br />
(MOROCCO) GOKALS LTD 4/14/2012<br />
SANOFI-AVENTIS<br />
(MOROCCO) GOKALS LTD 9/1/2013<br />
63<br />
MARCH 1, 2011
539<br />
540<br />
COARSUCAM<br />
TODDLER<br />
CO-ARTESUN (7 -<br />
13YRS)<br />
541 CO-ARTESUN ADULTS<br />
542 COBAXINE<br />
543 COBAXINE<br />
544 CO-DIOVAN<br />
545 CO-DIOVAN<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg/135<br />
mg Tablet Oral<br />
153 mg /50<br />
mg Tablet Oral<br />
153 mg / 50<br />
mg Tablet Oral<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple Syrup<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple<br />
HYDROCHLORTHI<br />
AZIDE+VALSARTA<br />
N 160/12.5 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
HYDROCHLORTHI<br />
AZIDE+VALSARTA<br />
N 80/12.5 mg Tablet Oral<br />
SANOFI-<br />
AVENTIS<br />
(COTE<br />
DIVIORE)<br />
GUILIN<br />
PHARMACEUT<br />
ICALS CO.<br />
LTD.<br />
GUILIN<br />
PHARMACEUT<br />
ICALS CO.<br />
LTD.<br />
BAFNA<br />
PHARMACEUT<br />
ICALS LTD<br />
BAFNA<br />
PHARMACEUT<br />
ICALS LTD<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
SCIENTIFIC<br />
OFFICE (NIG)<br />
NOVARTIS<br />
PHARMA AG<br />
SANOFI-AVENTIS<br />
(MOROCCO) GOKALS LTD 9/1/2013<br />
GUILIN<br />
PHARMACEUTICALS<br />
CO. LTD.<br />
GUILIN<br />
PHARMACEUTICALS<br />
CO. LTD.<br />
BAFNA<br />
PHARMACEUTICALS<br />
LTD<br />
BAFNA<br />
PHARMACEUTICALS<br />
LTD<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
GmbH<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 10/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 11/1/2011<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 11/1/2011<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 11/1/2011<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 6/11/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
64<br />
MARCH 1, 2011
546 COFFMED<br />
547 COLD TABLET<br />
548 COLDCAP<br />
549 COLDCARE<br />
550 COLDRID<br />
551 COLESTOP<br />
552 COLESTOP<br />
553 COMBAT-N<br />
DEXTROMETHORP<br />
HAN+GUAIFENISIN<br />
+PSEUDOEPHEDRI<br />
NE<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL<br />
10 mg + 200<br />
mg + 30 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Softgel)<br />
30 mg+4.0<br />
mg+ 500 mg Tablet Oral<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PHENYLEPHRIN<br />
E+OTHERS multiple Caplets oral<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PHENYLEPHRIN<br />
E+OTHERS Multiple Tablet Oral<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL Multiple Tablet Oral<br />
ATORVASTATIN<br />
CALCIUM 10 mg<br />
ATORVASTATIN<br />
CALCIUM 20 mg<br />
BETAMETHASONE<br />
+CLOTRIMAZOLE+<br />
NEOMYCIN Multiple<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Cream<br />
Topical<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
MERCURY<br />
HEALTHCARE<br />
PVT. LTD<br />
TRADE WINDS<br />
CHEMIST LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
TOBINCO<br />
PHARMACY<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
GEO MEDICORE<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
AGOG PHARMA<br />
LTD.<br />
TRADE WINDS<br />
CHEMIST LTD<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2012<br />
GEO MEDICORE<br />
LIMITED 8/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
PARMARTS<br />
PHARMACY LTD 9/1/2013<br />
TRADE WINDS<br />
CHEMIST LTD 4/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 9/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 9/1/2013<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
65<br />
MARCH 1, 2011
554 COMBIGAN<br />
555<br />
556<br />
COMPOUND SODIUM<br />
LACTATE I.V.<br />
COMPOUND SODIUM<br />
LACTATE I.V.<br />
557 COPAMOL<br />
BROMONIDINE+TI<br />
MOLOL 2 mg + 5 mg Eye drops<br />
COMPOUND<br />
SODIUM LACTATE Multiple<br />
COMPOUND<br />
SODIUM LACTATE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
CODEINE+PARACE<br />
TAMOL 500 mg/8 mg Tablet Oral<br />
558 COPEGUS RIBAVIRIN 200 mg Tablet Oral<br />
559 CO-PROXAMOL<br />
560 CO-PROXAMOL<br />
561 CO-PROXAMOL<br />
DEXTROPROPOXY<br />
PHENE+PARACETA 53.5mg/325<br />
MOL<br />
mg Tablet Oral<br />
DEXTROPROPOXY<br />
PHENE+PARACETA 53.5mg/325<br />
MOL<br />
mg Tablet Oral<br />
DEXTROPROPOXY<br />
PHENE+PARACETA 53.5mg/325<br />
MOL<br />
mg Tablet Oral<br />
562 CORATENOL P A ATENOLOL 50 mg Tablet Oral<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
FRESENIUS<br />
KABI INDIA<br />
PVT. LIMITED<br />
SIMRIT<br />
EXPORTS<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LABORATORI<br />
OS KENER SA<br />
ALLERGAN INC<br />
(usa)<br />
FRESENIUS KABI<br />
INDIA PVT.<br />
LIMITED<br />
FRESNIUS<br />
MAFATLAL<br />
MEDICALS LTD.<br />
STARWIN<br />
PRODUCTS LTD.<br />
F. HOFFMAN-LA<br />
ROCHE INC(USA)<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LABORATORIOS<br />
KENER SA<br />
BERNSWETT<br />
COMPANY LTD 4/1/2012<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2013<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ULTRAS<br />
PHARMACEUTIC<br />
ALS INC 3/1/2012<br />
66<br />
MARCH 1, 2011
563 CORBIS-H 10<br />
564 CORBIS-H 5<br />
BISOPROLOL +<br />
HYDROCHLOROTH<br />
IAZIDE<br />
BISOPROLOL +<br />
HYDROCHLOROTH<br />
IAZIDE<br />
10 mg + 6.25<br />
mg<br />
5 mg + 6.25<br />
mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
565 CORDARONE AMIODARONE 200 mg Tablet Oral<br />
566 CORORANGE IRON+VITAMINS Multiple Oral Drops<br />
567 CORORANGE PLUS<br />
568 CORRORANGE<br />
FOLIC<br />
ACID+IRON+VITAM<br />
IN B12 Multiple Syrup<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
569 CORYOL CARVEDILOL 25 mg Tablet Oral<br />
570 CORYOL CARVEDILOL 12.5 mg Tablet Oral<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
SANOFI<br />
WINTHROP<br />
LTD(UK)<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
OSON'S<br />
CHEMIST LTD<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
GR PHARMA<br />
LTD. 8/1/2013<br />
GR PHARMA<br />
LTD. 8/1/2013<br />
SANOFI WINTHROP<br />
LTD(UK) REISS & CO. 5/1/2012<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
OSON'S CHEMIST<br />
LTD 2/1/2012<br />
OSON'S CHEMIST<br />
LTD 5/1/2013<br />
OSON'S CHEMIST<br />
LTD 2/1/2012<br />
ATLAS<br />
PHARMACY<br />
LIMITED 11/1/2011<br />
ATLAS<br />
PHARMACY<br />
LIMITED 11/1/2011<br />
67<br />
MARCH 1, 2011
571 CORYOL CARVEDILOL 6.25 mg Tablet Oral<br />
572 CO-TENIDONE<br />
573 CO-TENIDONE<br />
574 CO-TRIMOXAZOLE<br />
575 CO-TRIMOXAZOLE<br />
576 CO-TRIMOXAZOLE<br />
577 CO-TRIMOXAZOLE<br />
578 CO-TRIMOXAZOLE<br />
579<br />
CO-TRIMOXAZOLE<br />
ORAL<br />
ATENOLOL+CHLO<br />
RTHALIDONE<br />
ATENOLOL+CHLO<br />
RTHALIDONE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
100 mg/25<br />
mg Tablet Oral<br />
50 mg/12.5<br />
mg Tablet Oral<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM 480 mg Tablet Oral<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM 480 mg Tablet Oral<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
200 mg/40<br />
mg Tablet Oral<br />
200mg/40<br />
mg/5mls<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM 240 mg/5 ml<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM 240 mg/5 ml<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
GEO MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
ATLAS<br />
PHARMACY<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 10/3/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
GEO MEDICORE<br />
LIMITED 12/31/2012<br />
GEO MEDICORE<br />
LIMITED 12/31/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
68<br />
MARCH 1, 2011
580 CPM<br />
CHLORPHENIRAMI<br />
NE 4 mg Tablet Oral<br />
581 CRESTOR ROSUVASTATIN 5 mg Tablet Oral<br />
582 CRESTOR ROSUVASTATIN 10 mg Tablet Oral<br />
583 CRESTOR ROSUVASTATIN 20 mg Tablet Oral<br />
584<br />
585<br />
586<br />
587<br />
588<br />
CRYSTALLINE<br />
PENICILLIN<br />
CRYSTALLINE<br />
PENICILLIN G<br />
CRYSTALLINE<br />
PENICILLIN G<br />
CRYSTALLINE<br />
PENICILLIN G<br />
CRYSTALLINE<br />
PENICILLIN INJECTION<br />
BENZYLPENICILLI<br />
N 1 MU<br />
BENZYLPENICILLI<br />
N 1,000,000 iu<br />
BENZYLPENICILLI<br />
N 1mega units<br />
BENZYLPENICILLI<br />
N 1,000,000 iu<br />
BENZYLPENICILLI<br />
N 1,000,000 I.U<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
CHINA<br />
NATIONAL<br />
PHARM.<br />
FOREIGN<br />
TRADE CORP.<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
SINOPHARM<br />
GH LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
IPR<br />
PHARMACEUTICALS<br />
INC.<br />
IPR<br />
PHARMACEUTICALS<br />
INC.<br />
IPR<br />
PHARMACEUTICALS<br />
INC.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
AGLOWMED<br />
LIMITED<br />
CHINA NATIONAL<br />
PHARM. FOREIGN<br />
TRADE CORP.<br />
TROGE MEDICAL<br />
GMBH<br />
CHINA NATIONAL<br />
PHARM. FOREIGN<br />
TRADE CORP.<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 11/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 5/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 5/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
UNICHEM<br />
GHANA LTD. 5/1/2013<br />
SINOPHARM GH<br />
LTD 5/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
ALOKEN<br />
PHARMACY 5/1/2013<br />
69<br />
MARCH 1, 2011
589 C-TRI T CEFTRIAXONE 1 g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
590 C-TRI T CEFUROXIME 250 mg Tablet Oral<br />
591 CUSICROM<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
SODIUM<br />
CROMOGLYCATE 2%w/v Eye drops ALCON CUSI ALCON CUSI<br />
592 CUSIMOLOL TIMOLOL 0.5%w/v Eye drops ALCON CUSI ALCON CUSI<br />
593 CYCLOKAPRON TRANEXAMIC ACID 100 mg/ ml<br />
594 CYFEN<br />
Injectable<br />
Solution<br />
CYPROHEPTADINE<br />
+MINERALS+PROT<br />
EIN+VITAMINS Multiple Syrup<br />
595 CYMBALTA DULOXETINE 30 mg<br />
596 CYMBALTA DULOXETINE 60 mg<br />
597 CYPROVITA<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
POKU<br />
PHARMA LTD.<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 10/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 5/1/2012<br />
PFIZER ITALIA S.r.<br />
L. GOKALS LTD 12/1/2012<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
POKU PHARMA<br />
LTD. 8/1/2012<br />
ELI LILLY AND<br />
(suisse) SA (<br />
Kenya) LILY S.A.(Spain) REISS & CO. 12/1/2012<br />
ELI LILLY AND<br />
(suisse) SA (<br />
Kenya) LILY S.A.(Spain) REISS & CO. 12/1/2012<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED 8/1/2013<br />
70<br />
MARCH 1, 2011
598 CYTOTEC MISOPROSTOL 200 mcg Tablet Oral<br />
599 D5 INFUSION GLUCOSE 5 g/ ml<br />
600 DAAMSENG FORTE<br />
601 DACLOGEL<br />
602 DAFLON 500<br />
603 DAKTACORT<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DICLOFENAC<br />
DIETHYLAMMONIU<br />
M 1.16%w/w Gel Topical<br />
DIOSMIN+HESPERI<br />
DIN<br />
HYDROCORTISONE<br />
+MICONAZOLE<br />
450 mg/50<br />
mg Tablet Oral<br />
2%w/w,<br />
1%w/w<br />
604 DAKTARIN CREAM MICONAZOLE 2 % w/w<br />
605 DALACIN C CLINDAMYCIN 150 mg<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PFIZER ITALIA<br />
S.r. L.<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
MERCURY<br />
HEALTHCARE<br />
PVT. LTD<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
SERVIER<br />
EGYPT<br />
INDUSTRY<br />
LTD<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
SEARLE DIVISON OF<br />
MONSANTO plc.<br />
(UK) GOKALS LTD 4/1/2011<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
SERVIER EGYPT<br />
INDUSTRY LTD<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
PFIZER PGM<br />
(FRANCE)<br />
GR PHARMA<br />
LTD. 12/1/2011<br />
DAAMASS CO.<br />
LIMITED 9/1/2011<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
OYSTER<br />
HEALTHCARE<br />
LTD. 4/1/2013<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 7/1/2011<br />
71<br />
MARCH 1, 2011
606 DALACIN C CLINDAMYCIN 10 mg/ml<br />
607 DALACIN C CLINDAMYCIN 150 mg/mls<br />
608 DALACIN T CLINDAMYCIN 1% w/v<br />
609 DALTA VITAMIN E 400 IU<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
Topical<br />
solution<br />
Capsule oral<br />
(Softgel)<br />
610 DAMANIL GLIBENCLAMIDE 5 mg Tablet Oral<br />
611 DANCIFLOX CIPROFLOXACIN 500 mg Tablet Oral<br />
612 DANEGAN<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
PFIZER ITALIA S.r.<br />
L.<br />
PFIZER<br />
MANUFACTURING<br />
PFIZER BELGIUM<br />
MANUFACTUR N.V.PFIZER<br />
ING BELGIUM MANUFACTURING<br />
N.V. BELGIUM NV<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
PHARMACIA &<br />
UPJOHN COMPANY<br />
(USA)<br />
STRIDES ARCOLAB<br />
LIMITED<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 11/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 4/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 8/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2013<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
DANDONG<br />
PHARMACEUTIC<br />
AL FACTORY 7/1/2012<br />
PROMETHAZINE<br />
HYDROCHLORIDE 5 mg/5mls Syrup DANNEX LTD DANNEX LTD DANNEX LTD 9/1/2012<br />
72<br />
MARCH 1, 2011
613 DANGYL METRONIDAZOLE 125 mg/5 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
614 DANGYL METRONIDAZOLE 200 mg Tablet Oral<br />
615 DANMETHER PDR<br />
616 DANNEX GRIPE WATER<br />
617 DANRUB<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
DILL OIL+SODIUM<br />
BICARBONATE<br />
CAPSICUM+METHY<br />
L SALICYLATE<br />
20 mg/120<br />
mg<br />
Granules For<br />
Oral Use<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 12/3/2011<br />
DANPONG<br />
PHARMACY 2/1/2013<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 12/31/2011<br />
0.06% + 50<br />
mg/5 ml Syrup DANNEX LTD DANNEX LTD DANNEX LTD 2/1/2013<br />
10%w/w +<br />
40%w/w<br />
618 DAOMAZ OMEPRAZOLE 20 mg<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 2/1/2013<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
619 DAONIL GLIBENCLAMIDE 5 mg Tablet Oral<br />
620 DAPRES LOPERAMIDE 2 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DAAMASS CO.<br />
LIMITED<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
AVENTIS PHARMA<br />
(PTY) LIMITED GOKALS LTD 8/1/2013<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
73<br />
MARCH 1, 2011
621 DARAZOLE<br />
622 DARTE-Q<br />
623 DARTE-Q<br />
PHTHALYLSULPHA<br />
THIAZOLE 500 mg Tablet Oral<br />
DIHYDROARTEMISI<br />
NIN+PIPERAQUINE<br />
DIHYDROARTEMISI<br />
NIN+PIPERAQUINE<br />
40 mg/320<br />
mg<br />
15 mg/ 120<br />
mg/ sachet<br />
624 DAZOLE METRONIDAZOLE 200 mg<br />
625 DAZOLE METRONIDAZOLE 200 mg/5 ml<br />
626 DECATYLEN<br />
627<br />
DEQUALINIUM +<br />
DIBUCAINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Granules For<br />
Oral Use<br />
Tablet film<br />
coated<br />
Oral<br />
Suspension<br />
DEEP FREEZE COLD<br />
GEL MENTHOL 2%w/w Gel Topical<br />
628 DEEP FREEZE SPRAY MENTHOL<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
KING-REPA<br />
TRADING (S)<br />
PTE LTD.<br />
KING-REPA<br />
TRADING (S)<br />
PTE LTD.<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
GOSUN PHARMA<br />
CORP.<br />
GOSUN PHARMA<br />
CORP.<br />
0.25 mg/0.03<br />
mg Lozenges MEPHA LTD. MEPHA LTD.<br />
40.%w/w/2%<br />
W/W<br />
Topical<br />
Spray<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
HEALTHCARE<br />
SERVICES LTD 7/1/2011<br />
HEALTHCARE<br />
SERVICES LTD 7/1/2011<br />
CASSEL<br />
RESEARCH CASSEL RESEARCH<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD PVT. LTD 8/1/2011<br />
CASSEL<br />
RESEARCH CASSEL RESEARCH<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD PVT. LTD 8/1/2011<br />
MENTHOLATU<br />
M COMPANY<br />
LTD<br />
MENTHOLATU<br />
M COMPANY<br />
LTD<br />
MENTHOLATUM<br />
COMPANY LTD<br />
MENTHOLATUM<br />
COMPANY LTD<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
GB PHARMA<br />
(GH) LTD 4/1/2013<br />
GB PHARMA<br />
(GH) LTD 12/31/2011<br />
74<br />
MARCH 1, 2011
629 DEEP HEAT RUB<br />
630 DEEP HEAT SPRAY<br />
631<br />
632<br />
633<br />
DEKLOFEN FORTE<br />
CAPLETS<br />
DELTA MEDICATED<br />
AND ANTISEPTIC<br />
DELTA PLUS<br />
MEDICATED AND<br />
ANTISEPTIC<br />
634 DEMOXIL PLUS-457.5<br />
635 DEPO-MEDROL<br />
636 DERMI COOL<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
HYDROXYETHYL<br />
SALICYLATE+MET<br />
HYL<br />
NICOTINATE+MET<br />
HYL SALICYLATE Multiple<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Topical<br />
Spray<br />
50 mg+ 500<br />
mg Tablet Oral<br />
TRICHLOROCARBA<br />
NILIDE 0.5%w/w Solid Soap<br />
TRICHLOROCARBA<br />
NILIDE+TRICLOSA<br />
N<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
0.5%w/w/0.0<br />
5%w/w Solid Soap<br />
400 mg +57.5<br />
mg<br />
METHYLPREDNISO<br />
LONE 40 mg/ml<br />
ZINC<br />
OXIDE+BORIC<br />
ACID+SALICYLIC<br />
ACID+0THERS Multiple<br />
Powder for<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
Powder for<br />
topical use<br />
MENTHOLATU<br />
M COMPANY<br />
LTD<br />
MENTHOLATU<br />
M COMPANY<br />
LTD<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
ORANGE<br />
WEST AFRICA<br />
GH. LTD<br />
ORANGE<br />
WEST AFRICA<br />
GH. LTD<br />
<strong>DRUG</strong><br />
INTERNATION<br />
AL LIMITED<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
PARAS<br />
PHARMACEUT<br />
ICALS LTD<br />
KNOLL<br />
PHARMACEUTICALS<br />
BASFIN<br />
CORPORATION<br />
MENTHOLATUM<br />
COMPANY LTD<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
ORANGE <strong>DRUG</strong>S<br />
LTD (NIGERIA)<br />
ORANGE <strong>DRUG</strong>S<br />
LTD (NIGERIA)<br />
<strong>DRUG</strong><br />
INTERNATIONAL<br />
LIMITED<br />
PFIZER WEST<br />
AFRICA (NIGERIA)<br />
PARAS<br />
PHARMACEUTICALS<br />
LTD<br />
GB PHARMA<br />
(GH) LTD 12/31/2011<br />
GB PHARMA<br />
(GH) LTD 12/31/2011<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/21/2015<br />
ORANGE WEST<br />
AFRICA GH. LTD 7/1/2011<br />
ORANGE WEST<br />
AFRICA GH. LTD 5/1/2012<br />
BENNYVEE<br />
CHEMISTS 12/31/2012<br />
PFIZER<br />
MANUFACTURIN<br />
G BELGIUM NV 4/1/2012<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
75<br />
MARCH 1, 2011
637 DERMIRON-PLUS<br />
BETAMETHASONE 0.05%w/w +<br />
+CLIOQUINOL+GE 0.1% w/w +<br />
NTAMYCIN+TOLNA 1% w/w + 1%<br />
FTATE<br />
w/w<br />
638 DERMOFIX SERTACONAZOLE 2% w/w<br />
639 DERMOVATE<br />
CLOBETASOL<br />
PROPIONATE 0.05%w/w<br />
640 DEXAMETHASONE DEXAMETHASONE 4mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
Injectable<br />
Solution<br />
641 DEXAMETHASONE DEXAMETHASONE 1 mg Tablet Oral<br />
642 DEXAMETHASONE DEXAMETHASONE 4mg/ml<br />
643 DEXATROL<br />
DEXAMETHASONE<br />
+NEOMYCIN+POLY<br />
MYXIN B Multiple<br />
Injectable<br />
Solution<br />
Ear/Eye<br />
Drops<br />
644 DEXEL GLIMEPIRIDE 2 mg Tablet Oral<br />
645 DEXEL GLIMEPIRIDE 4 mg Tablet Oral<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
FERRER<br />
INTERNACION<br />
AL S.A<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
FERRER<br />
INTERNACIONAL<br />
S.A<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 12/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. LEK SA<br />
EGYPTIAN<br />
INTERNATIONAL<br />
PHARMACEUTICAL<br />
IND. CO.<br />
RENIE CHEMISTS<br />
LIMITED 5/1/2012<br />
PALB<br />
PHARMACEUTIC<br />
ALS 5/1/2012<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. LEK SA 5/1/2011<br />
76<br />
MARCH 1, 2011
646 DEXEL GLIMEPIRIDE 3 mg Tablet Oral<br />
647 DEXEL GLIMEPIRIDE 1 mg Tablet Oral<br />
648 DEXONE DEXAMETHASONE 0.5 mg Tablet Oral<br />
649<br />
DEXORANGE<br />
HEMATINIC<br />
FOLIC<br />
ACID+IRON+VITAM<br />
IN B12 Multiple Syrup<br />
650 DIABEL METFORMIN 500 mg Tablet Oral<br />
651 DIABENIL GLIBENCLAMIDE 5 mg Tablet Oral<br />
652 DIABETALEX GLIBENCLAMIDE 5 mg Tablet Oral<br />
653 DIABETONE<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
654 DIALUM LOPERAMIDE 2 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. LEK SA 5/1/2011<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. LEK SA 5/1/2011<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
FRANCO-<br />
INDIAN<br />
PHARMACEUT<br />
ICALS PVT.<br />
LTD<br />
HILLS<br />
PHARMACY<br />
PHARMANOV<br />
A LTD.<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
FRANCO-INDIAN<br />
PHARMACEUTICALS<br />
PVT. LTD<br />
BELCO PHARMA<br />
PVT LTD<br />
ATLANTIC<br />
LABORATORIES<br />
CORP. LTD<br />
AGLOWMED<br />
LIMITED<br />
VITABIOTICS<br />
LTD. VITABIOTICS LTD.<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
REGENT<br />
PHARMACY 10/1/2012<br />
HILLS<br />
PHARMACY 7/1/2012<br />
PHARMANOVA<br />
LTD. 6/1/2011<br />
UNICHEM<br />
GHANA LTD. 12/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 9/1/2011<br />
ESKAY<br />
THERAPEUTICS 11/1/2013<br />
77<br />
MARCH 1, 2011
655 DIAMEN METFORMIN 500 mg<br />
656 DIAMICRON MR GLICLAZIDE 30 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Enteric<br />
Coated<br />
Tablet<br />
Modified<br />
Release<br />
657 DIAMOX ACETAZOLAMIDE 60 mg Caplets oral<br />
658 DIANE 35<br />
CYPROTERONE+E<br />
THINYLESTRADIOL 2, 0.35 mg Tablet Oral<br />
659 DIAZEKIN DIAZEPAM 5 mg Tablet Oral<br />
660 DIAZEKIN DIAZEPAM 10 mg Tablet Oral<br />
661 DIAZEPAM DIAZEPAM 5 mg/ml<br />
Injectable<br />
Solution<br />
662 DIAZEPAM DIAZEPAM 10 mg Tablet Oral<br />
663 DIAZEPAM DIAZEPAM 10 mg/vial<br />
664 DIAZEPAM DIAZEPAM 5 mg Tablet Oral<br />
665 DIAZEPAM DIAZEPAM 10 mg Tablet Oral<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT.<br />
LTD.<br />
SERVIER<br />
EGYPT<br />
INDUSTRY<br />
LTD<br />
ROCK<br />
CHEMISTS<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
HENAN TOPFOND<br />
PHARMACEUTICALS<br />
SERVIER EGYPT<br />
INDUSTRY LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2012<br />
OYSTER<br />
HEALTHCARE<br />
LTD. 4/1/2013<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
BAYER - SCHERING<br />
PHARMA<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
ROCK<br />
CHEMISTS<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
KINAPHARMA<br />
LTD 5/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
Injectable<br />
Solution PHARM-INTER PHARM-INTER<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 6/1/2011<br />
OSON'S CHEMIST<br />
LTD 12/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
78<br />
MARCH 1, 2011
666 DIAZEPAM DIAZEPAM 10 mg/2mll<br />
667 DIAZEPAM DIAZEPAM 5 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
668 DICLO-DENK 100 DICLOFENAC 100 mg Suppository<br />
669<br />
DICLO-DENK 100<br />
RETARD DICLOFENAC 100 mg<br />
670 DICLO-DENK 50 DICLOFENAC 50 mg<br />
671 DICLO-DENK 75 DICLOFENAC 75 mg/ vial<br />
672 DICLO-DENK EC 25 DICLOFENAC 25 mg<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
Tablet<br />
Enteric<br />
Coated<br />
673 DICLO-DOR DICLOFENAC 50 mg Tablet Oral<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
HAUPT PHARMA<br />
WULFING GmbH<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
HAUPT PHARMA<br />
WULFING GmbH<br />
DENK PHARMA<br />
GmbH & Co.<br />
KGDENK PHARMA<br />
GmbH & Co. KG<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 5/30/2013<br />
79<br />
MARCH 1, 2011
674 DICLO-DOR DICLOFENAC 25 mg/ml<br />
675 DICLOFENAC DICLOFENAC 75 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
676 DICLOFENAC DICLOFENAC 100 mg Suppository<br />
677 DICLOFENAC DICLOFENAC 50 mg<br />
Tablet<br />
Enteric<br />
Coated<br />
678 DICLOFENAC DICLOFENAC 50 mg Tablet Oral<br />
679 DICLOFENAC DICLOFENAC 100 mg Tablet Oral<br />
680 DICLOFENAC DICLOFENAC 50 mg Tablet Oral<br />
681 DICLOFENAC DICLOFENAC 75mg/5ml<br />
Injectable<br />
Solution<br />
682 DICLOFENAC DICLOFENAC 100 mg Suppository<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
COLORAMA<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
G. D. COOPER &<br />
CO. LIMITED<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
OKASA PHARMA<br />
PVT LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 6/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
TOBINCO<br />
PHARMACY 2/1/2012<br />
OSON'S CHEMIST<br />
LTD 5/1/2013<br />
OSON'S CHEMIST<br />
LTD 5/1/2013<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
SISHUI XIERKANG<br />
PHARMACEUTICAL<br />
COMPANY<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
80<br />
MARCH 1, 2011
683 DICLOFLAME DICLOFENAC 50 mg Tablet Oral<br />
684 DICLOFLAME-SR DICLOFENAC 100 mg<br />
685 DICLOKIN DICLOFENAC 50 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Enteric<br />
Coated<br />
686 DICLOLEX DICLOFENAC 100 mg Tablet Oral<br />
687 DICLOMAX DICLOFENAC 50 mg Tablet Oral<br />
688 DICLOPHARM DICLOFENAC 75 mg/3mls<br />
Injectable<br />
Solution<br />
689 DICLOVEN DICLOFENAC 100 mg Tablet Oral<br />
690 DICLOVEN DICLOFENAC 50 mg Tablet Oral<br />
691 DICLOWAL S 100 DICLOFENAC 100 mg Suppository<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
SRAVANI<br />
LABS LIMITED<br />
SRAVANI<br />
LABS LIMITED<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
SRAVANI LABS<br />
LIMITED<br />
SRAVANI LABS<br />
LIMITED<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
GR PHARMA<br />
LTD. 10/1/2011<br />
GR PHARMA<br />
LTD. 9/1/2012<br />
KINAPHARMA<br />
LTD 6/1/2011<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
OSON'S CHEMIST<br />
LTD 9/1/2011<br />
D. K. D.<br />
PHARMACY LTD 8/1/2013<br />
D. K. D.<br />
PHARMACY LTD 8/1/2013<br />
UNICHEM<br />
GHANA LTD. 8/1/2013<br />
81<br />
MARCH 1, 2011
692 DICLOWAL S 25 DICLOFENAC 25 mg Suppository<br />
693<br />
DICLOWAL S<br />
INFANTILE DICLOFENAC 12.50 mg Suppository<br />
694 DICONAC<br />
695 DICONAC<br />
696<br />
697<br />
DICLOFENAC<br />
POTASSIUM 50 mg<br />
DICLOFENAC<br />
POTASSIUM 100 mg<br />
DIDANOSINE<br />
CHEWABLE/DISPERSIB<br />
LE DIDANOSINE 200 mg<br />
DIDANOSINE<br />
CHEWABLE/DISPERSIB<br />
LE DIDANOSINE 100 mg<br />
698 DIFLAZON FLUCONAZOLE 150 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet<br />
Chewable<br />
Tablet<br />
Chewable<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
699 DIGOXIN DIGOXIN 1.25 mg Tablet Oral<br />
700 DIHYDROCODEINE DIHYDROCODEINE 30 mg Tablet Oral<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICHEM<br />
GHANA LTD. 10/1/2011<br />
UNICHEM<br />
GHANA LTD. 10/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
ATLAS<br />
PHARMACY<br />
LIMITED 12/31/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
82<br />
MARCH 1, 2011
701 DIHYDROCODEINE DIHYDROCODEINE 30 mg Tablet Oral<br />
702 DIKLOGINE<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg + 500<br />
mg Tablet Oral<br />
703 DILATREND CARVEDILOL 25 mg Tablet Oral<br />
704 DILATREND CARVEDILOL 12.5 mg Tablet Oral<br />
ROCK<br />
CHEMISTS<br />
MERCURY<br />
HEALTHCARE<br />
PVT. LTD<br />
F. HOFFMAN<br />
LA-ROCHE<br />
S.p.A. (Italy)<br />
F. HOFFMAN<br />
LA-ROCHE<br />
S.p.A. (Italy)<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN LA-<br />
ROCHE S.p.A.<br />
(Italy)F.<br />
HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN LA-<br />
ROCHE S.p.A.<br />
(Italy)F.<br />
HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
PARMARTS<br />
PHARMACY LTD 4/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 12/1/2012<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 10/1/2011<br />
83<br />
MARCH 1, 2011
705 DILATREND CARVEDILOL 6.25 mg Tablet Oral<br />
706 DILTIAZEM DILTIAZEM 120 mg Tablet Oral<br />
707 DILTIAZEM DILTIAZEM 120 mg Tablet Oral<br />
708 DIOVAN VALSARTAN 80 mg Tablet Oral<br />
709<br />
710<br />
DIPHEX ANTITUSSIVE<br />
& EXPECTORANT<br />
COUGH<br />
DIPHEX<br />
BRONCHODILATOR &<br />
MUCOLYTIC COUGH<br />
AMMONIUM<br />
CHLORIDE+BROM<br />
HEXINE+DEXTROM<br />
ETHORPHAN+MEN<br />
THOL<br />
BROMHEXINE+GU<br />
AIAPHENESIN+ME<br />
NTHOL+TERBUTA<br />
LINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
4 mg+5<br />
mg+50<br />
mg+2.5 mg Syrup<br />
4 mg/50<br />
mg/2.5<br />
mg/1.25<br />
mg/5 ml Syrup<br />
F. HOFFMAN<br />
LA-ROCHE<br />
S.p.A. (Italy)<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
NOVARTIS<br />
PHARMA AG<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN LA-<br />
ROCHE S.p.A.<br />
(Italy)F.<br />
HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
NOVARTIS PHARMA<br />
GmbH<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 12/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2011<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 8/1/2012<br />
84<br />
MARCH 1, 2011
711<br />
DIPHEX JUNIOR<br />
COUGH<br />
DIPHENHYDRAMIN<br />
E+MENTHOL+SODI<br />
UM CITRATE<br />
712 DIPRIVAN PROPOFOL 10 mg/ml<br />
713 DIPRIVAN PROPOFOL 20 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
7mg +<br />
0.55mg +<br />
28.5mg/5ml Syrup<br />
714 DIPROFOS BETAMETHASONE 5mg + 2 mg<br />
715 DITHRANOL DITHRANOL 0.1% w/w<br />
716 DNS INFUSION<br />
717<br />
DNS INTRAVENOUS<br />
INFUSION<br />
GLUCOSE+SODIUM<br />
CHLORIDE<br />
GLUCOSE+SODIUM<br />
CHLORIDE<br />
5 g, 0.9g/100<br />
ml<br />
5%w/v/0.9%<br />
w/v<br />
718 DOBUTAMINE DOBUTAMINE 250 mg/5 ml<br />
719 DOLONOL<br />
Injectable<br />
emulsion<br />
Injectable<br />
emulsion<br />
Injectable<br />
Suspension<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
SCHERING-<br />
PLOUGH<br />
(PTY)<br />
LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
CORDEN PHARMA<br />
SPA<br />
CORDEN PHARMA<br />
SPA<br />
SCHERING-PLOUGH<br />
LABO<br />
N.V.(BELGIUM)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 8/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 8/1/2013<br />
GOKALS-<br />
LABOREX LTD 7/1/2013<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 11/1/2012<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL Multiple Tablet Oral<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
GR PHARMA<br />
LTD. 12/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
ESKAY<br />
THERAPEUTICS 7/1/2012<br />
85<br />
MARCH 1, 2011
720 DOLOPLUS<br />
721 DOLOTAB<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
722 DOMADOL TRAMADOL 100 mg<br />
723 DOPAMINE DOPAMINE 200 mg/5ml<br />
724 DORIBAX<br />
DORIPENEM<br />
MONOHYDRATE 500 mg<br />
725 DORMICUM MIDAZOLAM 5 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg/500<br />
mg Tablet Oral<br />
325 mg/400<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Powder<br />
Injectable<br />
Solution<br />
726 DORMICUM MIDAZOLAM 15 mg Tablet Oral<br />
727 DORMICUM MIDAZOLAM 5 mg/ml<br />
Injectable<br />
Solution<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
JANSSEN-<br />
CILAG<br />
(LEBANON)<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
SHIONOGI &<br />
COMPANY LTD<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
ESKAY<br />
THERAPEUTICS 5/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
GR PHARMA<br />
LTD. 8/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2012<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
86<br />
MARCH 1, 2011
728 DOSTINEX CABERGOLINE 0.50 mg Tablet Oral<br />
729 DOXAZOSIN DOXAZOSIN 4 mg Tablet Oral<br />
730 DOXAZOSIN DOXAZOSIN 2 mg Tablet Oral<br />
731<br />
DOXORUBICIN<br />
HYDROCHLORIDE DOXORUBICIN 50 mg/ Vial<br />
732 DOXYCIL-100 DOXYCYCLINE 100 mg<br />
733 DOXYCYCLINE DOXYCYCLINE 100 mg<br />
734 DOXYCYCLINE DOXYCYCLINE 100 mg<br />
735 DOXYCYCLINE DOXYCYCLINE 100 mg<br />
736 DOXYKAM DOXYCYCLINE 100 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROCK<br />
CHEMISTS<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
PHARMACIA &<br />
UPJOHN INT<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
PFIZER GLOBAL<br />
PHARMACEUTIC<br />
ALS (NIGERIA) 4/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
DEVATS INDIA PVT<br />
LTD ROCK CHEMISTS 2/1/2012<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
87<br />
MARCH 1, 2011
737 DOXYKIN DOXYCYCLINE 100 mg<br />
738 DOXYLAG DOXYCYCLINE 100 mg<br />
739 DRAGON<br />
LIDOCAINE+VITAM<br />
IN E 10% + 1%<br />
740 DROPERIDOL DROPERIDOL 2.5 mg<br />
741 DUMOCALCIUM<br />
CALCIUM+VITAMIN<br />
D<br />
742 DUOCEF CEFTRIAXONE 1 g<br />
743 DUO-COTECXIN<br />
744 DUOTRAV<br />
DIHYDROARTEMISI<br />
NIN+PIPERAQUINE<br />
TIMOLOL+TRAVOP<br />
ROST<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Topical<br />
Spray<br />
Injectable<br />
Solution<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
JEWIN<br />
PHARMA<br />
(SHANDONG)<br />
CO. LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD 8/1/2013<br />
JEWIN PHARMA<br />
(SHANDONG) CO.<br />
LTD 12/31/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
430 mg/500<br />
iu Tablet Oral DANNEX LTD DANNEX LTD DANNEX LTD 9/1/2012<br />
Injectable<br />
Suspension<br />
40 mg/320<br />
mg Tablet Oral<br />
5 mg/40<br />
mcg/ml Eye drops<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT.<br />
LTD.<br />
BEIJING<br />
HOLLEY-<br />
COTEC<br />
PHARMACEUT<br />
ICALS CO.<br />
LTD.<br />
ALCON-<br />
COUVREUR<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
BEIJING HOLLEY-<br />
COTEC<br />
PHARMACEUTICALS<br />
CO. LTD.<br />
ALCON-<br />
COUVREUR<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2012<br />
LANSAH<br />
CHEMISTS LTD 5/1/2013<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 11/1/2012<br />
88<br />
MARCH 1, 2011
745 DURACAPS<br />
746 DUROL TONIC<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
747 DUROMINE PHENTERMINE 30 mg<br />
25 mg + 200<br />
mg + 325 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PERFECT<br />
PHARMACEUT<br />
ICALS LTD<br />
PERFECT<br />
PHARMACEUTICALS<br />
LTD<br />
PERFECT<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
FOLIC<br />
ACID+IRON+MULTI<br />
VITAMINS Multiple Syrup DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2012<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
748 DYCLOMAX DICLOFENAC 50 mg Tablet Oral<br />
749<br />
ECL BABY TEETHING<br />
MIXTURE<br />
PARACETAMOL+Q<br />
UININE Mixtures<br />
750 ECL MULTIVITAMIN MULTIVITAMIN Multiple Tablet Oral<br />
751 ECL MULTIVITAMIN MULTIVITAMIN Multiple Syrup<br />
752<br />
ECL VITAMIN B<br />
COMPLEX<br />
753 ECONAZINE<br />
VITAMIN B<br />
COMPLEX Multiple Syrup<br />
ECONAZOLE+TRIA<br />
MCINOLONE<br />
10.0 mg+1<br />
mg / g<br />
Cream<br />
Topical<br />
3M<br />
PHARMACEUT<br />
ICALS<br />
(AUSTRALIA)<br />
PVT LTD<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
3M<br />
PHARMACEUTICALS<br />
(AUSTRALIA) PVT<br />
LTD<br />
SMITHKLINE<br />
BEECHAM INT.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
UNICHEM<br />
GHANA LTD. 12/31/2011<br />
GLAXOSMITHKLI<br />
NE UK LIMITED 9/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
89<br />
MARCH 1, 2011
754 ECONOPRED PLUS PREDNISOLONE 1%w/v Eye drops<br />
755 EFAVIRENZ EFAVIRENZ 50 mg<br />
756 EFAVIRENZ EFAVIRENZ 100 mg<br />
757 EFAVIRENZ EFAVIRENZ 200 mg<br />
758 EFAVIRENZ EFAVIRENZ 600 mg<br />
759 EFAVIRENZ EFAVIRENZ 200 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
760 EFFERVEN-C VITAMIN C 1000 mg Tablet Oral<br />
761 ELERON<br />
762 ELERON<br />
IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
250 mg/ 5<br />
mls Syrup<br />
100 mg/ 550<br />
mcg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
ALCON<br />
LABORATORIE<br />
S INC (USA)<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
ALCON<br />
LABORATORIES INC<br />
(USA)<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
GENO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
HILLS<br />
PHARMACY<br />
STRIDES ARCOLAB<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
GENO<br />
PHARMACEUTICALS<br />
LIMITED<br />
GENO<br />
PHARMACEUTICALS<br />
LIMITED<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 10/1/2013<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
ROXIN GHANA<br />
LTD 11/1/2013<br />
HILLS<br />
PHARMACY 8/1/2011<br />
HILLS<br />
PHARMACY 8/1/2012<br />
90<br />
MARCH 1, 2011
763 EMGIFLOX FLUCLOXACILLIN 250 mg<br />
764 EMGIKOF-D<br />
765 EMGIKOF-S<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DEXTROMETHORP<br />
HAN<br />
HYDROBROMIDE+P<br />
HENYLEPHRINE<br />
HYDROCHLORIDE+<br />
CE Multiple Syrup<br />
AMBROXOL+SALB<br />
UTAMOL+GUIAFEN<br />
ESIN+RACEMENTH<br />
OL Multiple Syrup<br />
766 EMGIMYCIN ERYTHROMYCIN 250 mg Tablet Oral<br />
767 EMGIPINE NIFEDIPINE 10 mg<br />
Capsule<br />
Extended<br />
Release<br />
768 EMGIPROFEN IBUPROFEN 400 mg Tablet Oral<br />
769 EMGIPROFEN IBUPROFEN 200 mg Tablet Oral<br />
770 EMGIVIT MULTIVITAMIN Multiple<br />
Tablet film<br />
coated<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
91<br />
MARCH 1, 2011
771<br />
EMULSIFYING<br />
OINTMENT<br />
EMULSIFYING<br />
OINTMENT 30 %w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
772 ENACEF CEFUROXIME 125 mg Tablet Oral<br />
773 ENACEF CEFUROXIME 250 mg Tablet Oral<br />
774 ENAFEN IBUPROFEN 100 mg/5 mls<br />
Oral<br />
Suspension<br />
775 ENALAPRIL ENALAPRIL 5 mg Tablet Oral<br />
776 ENALAPRIL ENALAPRIL 10 mg Tablet Oral<br />
777 ENALAPRIL ENALAPRIL 20 mg Tablet Oral<br />
778 ENALAPRIL ENALAPRIL 5 mg Tablet Oral<br />
779 ENALAPRIL ENALAPRIL 10 mg Tablet Oral<br />
780 ENALAPRIL ENALAPRIL 20 mg Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
92<br />
MARCH 1, 2011
781 ENALAPRIL ENALAPRIL 20 mg Tablet Oral<br />
782 ENALAPRIL ENALAPRIL 5 mg Tablet Oral<br />
783 ENALAPRIL ENALAPRIL 10 mg Tablet Oral<br />
784 ENAMYCIN DPFS ERYTHROMYCIN 125 mg/5 mls<br />
785 ENAT VITAMIN E 400 IU<br />
786 ENGERIX-B ADULT HEPATITIS B 20 mcg/ ml<br />
787<br />
ENGERIX-B<br />
PEADIATRIC HEPATITIS B<br />
788 ENHANCIN<br />
789 ENHANCIN 625<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 1.2 g<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
10 mcg/0.5<br />
ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Softgel)<br />
Injectable<br />
Suspension<br />
Injectable<br />
Suspension<br />
Injectable<br />
Powder<br />
500 mg/125<br />
mg Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
GLAXOSMITHKLINE<br />
BIOLOGICALS S.<br />
A.<br />
GLAXOSMITHKLINE<br />
BIOLOGICALS S.<br />
A.<br />
RANBAXY RANBAXY<br />
LABORATORIE LABORATORIES<br />
S LTD. LTD.<br />
RANBAXY RANBAXY<br />
LABORATORIE LABORATORIES<br />
S LTD. LTD.<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 7/1/2011<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD 9/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
GB PHARMA<br />
(GH) LTD 10/1/2012<br />
GB PHARMA<br />
(GH) LTD 9/1/2011<br />
93<br />
MARCH 1, 2011
790 ENOFEN PM<br />
791 ENTACYD PLUS<br />
792<br />
EPHEDRINE<br />
HYDROCHLORIDE<br />
ACETAMINOPHEN+<br />
DIPHENHYDRAMIN<br />
E HCL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg+25<br />
mg Caplets oral<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS 4 g + 8 g + 2g<br />
EPHEDRINE<br />
HYDROCHLORIDE 50 mg/ml<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
793 EPICIPRIN CIPROFLOXACIN 3mg Eye drops<br />
794 EPICROM<br />
SODIUM<br />
CROMOGLYCATE 2% w/v Eye drops<br />
795 EPIFENAC DICLOFENAC 0.1% w/v Eye drops<br />
796 EPILIM<br />
VALPROATE<br />
SODIUM 200mg/5ml Syrup<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
SQUARE<br />
PHARMACEUT<br />
ICALS<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
SQUARE<br />
PHARMACEUTICALS<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 7/1/2011<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 8/1/2013<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS 2/1/2012<br />
EGYPTIAN<br />
INTERNATION EGYPTIAN<br />
AL<br />
INTERNATIONAL<br />
PHARMACEUT PHARMACEUTICAL<br />
ICAL IND. CO. IND. CO.<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
SANOFI<br />
WINTHROP<br />
LTD(UK)<br />
EGYPTIAN<br />
INTERNATIONAL<br />
PHARMACEUTICAL<br />
IND. CO.<br />
EGYPTIAN<br />
INTERNATIONAL<br />
PHARMACEUTICAL<br />
IND. CO.<br />
SANOFI<br />
WINTHROP(France)<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
REISS & CO. 9/1/2011<br />
94<br />
MARCH 1, 2011
797 EPITIMOL TIMOLOL 0.5%w/v Eye drops<br />
798 EQUIRAB<br />
ANTIRABIES<br />
SERUM 1000 iu<br />
799 ERGOMETRINE ERGOMETRINE 0.5 mg/ml<br />
800 ERYCIN 125 ERYTHROMYCIN 125 mg/5 mls<br />
801 ERYTHROKIN ERYTHROMYCIN 125 mg/5 ml<br />
802 ERYTHROLEX ERYTHROMYCIN 125 mg/5 ml<br />
803 ESCOLD<br />
804 ESCOTRIM<br />
805 ESIDREX<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PSEUDOEPHEDR<br />
INE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
EGYPTIAN<br />
INTERNATIONAL<br />
PHARMACEUTICAL<br />
IND. CO.<br />
BHARAT<br />
PARENTERALS BHARAT<br />
LTD PARENTERALS LTD<br />
Injectable<br />
Solution PHARM-INTER PHARM-INTER<br />
Oral<br />
Suspension PHARM-INTER PHARM-INTER<br />
Powder for<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
500 mg/2<br />
mg/30 mg Tablet Oral<br />
SULPAHMETHOXA<br />
ZOLE+TRIMETHOP<br />
RIM 480 mg Tablet Oral<br />
HYDROCHLORTHI<br />
AZIDE 25.00 mg Tablet Oral<br />
806 ESKABEN ALBENDAZOLE 400 mg<br />
Tablet<br />
Chewable<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
AGLOWMED<br />
LIMITED<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE<br />
S.A.<br />
NOVARTIS PHARMA<br />
AG<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
RENIE CHEMISTS<br />
LIMITED 2/1/2013<br />
FAR EAST<br />
MERCANTILE 7/1/2013<br />
OSON'S CHEMIST<br />
LTD 11/1/2012<br />
KINAPHARMA<br />
LTD 9/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
UNICHEM<br />
GHANA LTD. 7/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
ESKAY<br />
THERAPEUTICS 12/31/2011<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
ESKAY<br />
THERAPEUTICS 4/1/2013<br />
95<br />
MARCH 1, 2011
807 ESKAFULVIN GRISEOFULVIN 125 mg Tablet Oral<br />
808 ESKAGYL METRONIDAZOLE 200 mg Tablet Oral<br />
809 ESKAGYL-DS METRONIDAZOLE 400 mg Tablet Oral<br />
810 ESKAM PIROXICAM 20 mg<br />
811 ESKAMIDE LOPERAMIDE 2 mg<br />
812 ESKAPIL<br />
CAFFEINE+EPHED<br />
RINE<br />
HCL+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
30 mg+15<br />
mg+ 500 mg Tablet Oral<br />
813 ESKAZEPAM DIAZEPAM 5 mg Tablet Oral<br />
814 ESKYPAN TABLET<br />
HYOSCINE<br />
BUTYLBROMIDE 10 mg Tablet Oral<br />
815 EURAX CROTAMITON 10 %w/w<br />
Cream<br />
Topical<br />
816 EUROVIT MULTIVITAMIN Multiple Oral Drops<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
BETA<br />
HEALTHCARE<br />
GB PHARMA<br />
(uk) LTD.<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
MISSION VIVACARE<br />
LIMITED<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
ESKAY<br />
THERAPEUTICS 3/1/2013<br />
ESKAY<br />
THERAPEUTICS 9/1/2011<br />
ESKAY<br />
THERAPEUTICS 7/1/2012<br />
ESKAY<br />
THERAPEUTICS 9/1/2013<br />
ESKAY<br />
THERAPEUTICS 7/1/2011<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
ESKAY<br />
THERAPEUTICS 3/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 1/10/2012<br />
GB PHARMA<br />
(GH) LTD 4/1/2012<br />
96<br />
MARCH 1, 2011
817 EUROVIT<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
818 EUSOL LOTION EUSOL LOTION BP<br />
819 EXADUN<br />
820 EXFORGE<br />
821 EXFORGE<br />
CAFFEINE+PARAC<br />
ETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Topical<br />
solution<br />
32.5 mg +<br />
325 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5/160 mg Tablet Oral<br />
AMLODIPINE+VALS<br />
ARTAN 10/160 mg Tablet Oral<br />
822 EXICAM 20 PIROXICAM 20 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
823 EXJADE DEFERASIROX 500 mg Tablet Oral<br />
824 EXJADE DEFERASIROX 125 mg Tablet Oral<br />
825 EXJADE DEFERASIROX 250 mg Tablet Oral<br />
826 EXOCIN OFLOXACIN 3mg/ml Eye drops<br />
GB PHARMA<br />
(GH) LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
TOBINCO<br />
PHARMACY<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
MISSION VIVACARE<br />
LIMITED<br />
KAMA INDUSTRIES<br />
LTD.<br />
GB PHARMA<br />
(GH) LTD 10/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
NOVARTIS PHARMA<br />
AG 12/1/2012<br />
NOVARTIS PHARMA<br />
AG 12/1/2012<br />
NINGBO<br />
DAHONGYING<br />
PHARMACEUTICAL<br />
CO. LTD<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 3/1/2013<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 3/1/2013<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 4/6/2013<br />
ALLERGAN<br />
PHARMACEUTICALS BERNSWETT<br />
COMPANY LTD 12/1/2012<br />
97<br />
MARCH 1, 2011
827 EXPECTOLYN<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE+ME<br />
NTHOL+SODIUM<br />
CITRATE<br />
828 EXTACEF 200 DT CEFIXIME 200 MG<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
135 mg/14<br />
mg/1.25<br />
mg/57 mg Syrup<br />
Tablet<br />
Dispersible<br />
829 EZY-LAX BISACODYL 5 mg Tablet Oral<br />
830 F.D.<br />
831<br />
832<br />
833<br />
FALCIMON KIT<br />
(ADOLESCENT)<br />
FALCIMON KIT<br />
(ADULTS)<br />
FALCIMON KIT<br />
(CHILDREN)<br />
834 FANSIDAR<br />
ADRENALINE+LIGN<br />
OCAINE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
2%<br />
w/v/1:100,0<br />
00 units<br />
Injectable<br />
Solution<br />
50 mg/153<br />
mg Tablet Oral<br />
50 mg/200<br />
mg Tablet Oral<br />
50 mg/153<br />
mg Tablet Oral<br />
PYRIMETHAMINE+<br />
SULPHADOXINE 525 mg Tablet Oral<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
STARWIN<br />
PRODUCTS LTD.<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2012<br />
BLUE CROSS<br />
LABORATORIE BLUE CROSS<br />
S<br />
LABORATORIES ROCK CHEMISTS 9/1/2012<br />
ESKAY<br />
THERAPEUTIC<br />
S 5/1/2013<br />
ADAM<br />
DENTAL AND<br />
MEDICAL<br />
WORLD<br />
COMPANY<br />
LTD<br />
LABORATORIOS<br />
ZEYCO, S.A. DE<br />
C.V.<br />
ADAM DENTAL<br />
AND MEDICAL<br />
WORLD<br />
COMPANY LTD 7/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 3/10/2012<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 3/10/2012<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 3/10/2012<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
F. HOFFMAN LA-<br />
ROCHE PRODUCTS<br />
(PTY) LTD. (SOUTH<br />
AFRICA)<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 5/1/2012<br />
98<br />
MARCH 1, 2011
835 FANTEM<br />
836 FASIPRO<br />
837 FEFOL<br />
838 FELITONE<br />
839 FENAMOL<br />
840 FENBASE EXTRA<br />
841 FENIC<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
CIPROFLOXACIN+<br />
TINIDAZOLE<br />
FERROUS<br />
SULPHATE+FOLIC<br />
ACID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
20 mg+120<br />
mg Tablet Oral<br />
600 mg/500<br />
mg Tablet Oral<br />
150 mg,/500<br />
mcg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
500 mg/400<br />
mg Caplets oral<br />
30 mg + 400<br />
mg + 500 mg Tablet Oral<br />
MEDINOMICS<br />
HEALTHCARE<br />
PRIVATE LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
FELIBEN<br />
PHARMACY<br />
LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup NIC PHARMA<br />
842 FENISTIL DIMETHINDENE 0.05 ml/drop Eye drops<br />
BETA<br />
HEALTHCARE<br />
MADRAS<br />
PHARMACEUTICALS DAAMASS CO.<br />
LIMITED 6/1/2011<br />
MISSION VIVACARE<br />
LIMITED<br />
MEDREICH<br />
LIMITED<br />
NAVKETAN<br />
PHARMA PVT. LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
GALPHA<br />
LABORATORIES<br />
LIMITED<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
GB PHARMA<br />
(GH) LTD 4/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2013<br />
FELIBEN<br />
PHARMACY<br />
LIMITED 6/1/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
BASE PHARMACY<br />
LIMITED 3/1/2013<br />
SALOM<br />
PHARMACY<br />
LIMITED 7/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
99<br />
MARCH 1, 2011
843 FENPAR<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
844 FENTANYL-FRESENIUS FENTANYL<br />
845 FENTANYL-JANSSEN FENTANYL<br />
846 FEROCLEAR<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
847 FERROFORCE FOLIC ACID+IRON<br />
848 FERROLEX<br />
849 FERROMEX<br />
850 FERROSTRANE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
400 mg/325<br />
mg Tablet Oral<br />
100 mcg/2<br />
mls<br />
100 mcg/2<br />
mls<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
50 mg/0.5<br />
mg/5 ml Syrup<br />
200 mg/25<br />
mg Tablet Oral<br />
GLYCEROPHOSPH<br />
ATE+VITAMINS+IR<br />
ON Multiple Syrup<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
ROKMER<br />
PHARMA<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
MEDREICH<br />
LIMITED<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
AGOG PHARMA<br />
LTD.MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
AGLOWMED<br />
LIMITED<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 10/1/2011<br />
ROKMER<br />
PHARMA<br />
LIMITED 11/1/2012<br />
UNICHEM<br />
GHANA LTD. 4/1/2011<br />
UNICHEM<br />
GHANA LTD. 3/1/2013<br />
FERROUS<br />
FUMARATE 200 mg Tablet Oral DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2012<br />
FERROUS SODIUM<br />
EDETATE 0.68% Syrup<br />
PFIZER<br />
AFRIQUE,<br />
DAKAR<br />
SENEGAL<br />
PFIZER AFRIQUE,<br />
DAKAR SENEGAL<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 7/1/2011<br />
100<br />
MARCH 1, 2011
851 FERROTONE<br />
852 FERROUS FUMARATE<br />
853 FERROUS SULPHATE<br />
854 FERUP<br />
855 FIFOLAR<br />
FOLIC<br />
ACID+IRON+VITAM<br />
IN B12 Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
FERROUS<br />
FUMARATE 200 mg Tablet Oral<br />
FERROUS<br />
SULPHATE 200 mg Tablet Oral<br />
FOLIC<br />
ACID+IRON+MULTI<br />
VITAMINS Multiple Syrup<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple<br />
Oral<br />
Suspension<br />
856 FINASTERIDE FINASTERIDE 5 mg Tablet Oral<br />
857 FINASTERIDE FINASTERIDE 5 mg Tablet Oral<br />
858 FINMOL<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
859 FISHERMAN'S FRIEND PEPPERMINT OIL Multiple Lozenges<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED<br />
(INDIA)<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED (INDIA)<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
TABLETS (INDIA)<br />
LIMITED<br />
LARK LARK<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
50 mg/500<br />
mg Caplets oral GVS LABS GVS LABS<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LOFTHOUSE OF<br />
FLEETWOOD LTD<br />
OSON'S CHEMIST<br />
LTD 10/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
DAAMASS CO.<br />
LIMITED 9/1/2011<br />
BIG MARON<br />
PHARMACY 3/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
TOBINCO<br />
PHARMACY 7/18/2013<br />
UNICHEM<br />
GHANA LTD. 12/1/2012<br />
101<br />
MARCH 1, 2011
860 FLAGENTYL SECNIDAZOLE 500 mg Tablet Oral<br />
861 FLAGYL METRONIDAZOLE 500 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ovule<br />
Vaginal<br />
862 FLAGYL METRONIDAZOLE 400 mg Tablet Oral<br />
863 FLAGYL METRONIDAZOLE 200 mg/5 mls<br />
864 FLAREX<br />
865 FLATRO METRONIDAZOLE<br />
866 FLEXY<br />
Oral<br />
Suspension<br />
FLUOROMETHOLO<br />
NE 0.1%w/v Eye drops<br />
500 mg/100<br />
ml<br />
Injectable<br />
Solution<br />
DICLOFENAC<br />
DIETHYLAMMONIU<br />
M 1g/1.16 g Gel Topical<br />
SANOFI<br />
AVENTIS<br />
NIGERIA<br />
LIMITED<br />
SANOFI<br />
AVENTIS<br />
FRANCE<br />
SANOFI<br />
AVENTIS<br />
NIGERIA<br />
LIMITED<br />
RHONE-POULENC<br />
RORER (FRANCE) GOKALS LTD 8/1/2013<br />
HAUPT PHARMA<br />
LIVRON<br />
GOKALS-<br />
LABOREX LTD 8/1/2013<br />
MAY & BAYKER<br />
NIGERIA PLC GOKALS LTD 8/1/2013<br />
RHONE-<br />
POULENC<br />
RORER SA<br />
(PTY)<br />
LTD(SOUTH<br />
AFRICA) MAY & BAKER LTD GOKALS LTD 8/1/2013<br />
ALCON-<br />
COUVREUR<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
ALCON-<br />
COUVREUR<br />
MISSION VIVACARE<br />
LIMITED<br />
T.P. <strong>DRUG</strong><br />
LABORATORIES<br />
(1969) CO., LTD<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
OSON'S CHEMIST<br />
LTD 12/31/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 3/1/2012<br />
102<br />
MARCH 1, 2011
867 FLIXOTIDE FLUTICASONE 50 mcg<br />
868 FLOXA FLUCLOXACILLIN 250 mg<br />
869 FLOXAZOLE<br />
CIPROFLOXACIN+<br />
TINIDAZOLE<br />
500 mg + 400<br />
mg<br />
870 FLUCIGAN FLUCONAZOLE 150 mg<br />
871 FLUCOR DAY<br />
872 FLUCOR NIGHT<br />
DEXTROMETHORP<br />
HAN+PARACETAM<br />
OL+PSEUDOEPHED<br />
RINE Multiple<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PSEUDOEPHEDR<br />
INE<br />
250 mg/2<br />
mg/30 mg<br />
873 FLUKA-150 FLUCONAZOLE 150 mg<br />
874 FLUMYC-150 FLUCONAZOLE 150 mg<br />
875 FLUOXETINE FLUOXETINE 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Inhalational<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet<br />
Dispersible<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
BRAVO<br />
HEALTHCARE<br />
LTD<br />
AMBICA<br />
PHARMA<br />
SALES<br />
GLAXOWELLCOME<br />
PRODUCTION<br />
(FRANCE)<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
BRAVO<br />
HEALTHCARE LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
BEDITA<br />
PHARMACY HOVID Bhd.<br />
BEDITA<br />
PHARMACY HOVID Bhd.<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
BRAVO<br />
HEALTHCARE<br />
LTD 11/1/2012<br />
SPINTEX<br />
CHEMIST LTD 4/1/2011<br />
BEDITA<br />
PHARMACY 12/31/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 10/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
103<br />
MARCH 1, 2011
876<br />
FLUPHENAZINE<br />
DECANOATE FLUPHENAZINE 25 mg/ml<br />
877 FLUREST<br />
PARACETAMOL+PS<br />
EUDOEPHEDRINE+<br />
TRIPOLIDONE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
500 mg/60<br />
mg/2.5 mg Tablet Oral<br />
878 FLUTAMIDE FLUTAMIDE 250 mg Tablet Oral<br />
879 FLUTAMIDE FLUTAMIDE 250 mg Tablet Oral<br />
880 FLUXAKIN FLUCLOXACILLIN 125 mg/5 ml<br />
881 FLUZA FLUCONAZOLE 150 mg<br />
882 FML-NEO<br />
FLUOROMETHOLO<br />
NE+NEOMYCIN<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
0.1%w/w/0.5<br />
%w/w Eye drops<br />
883 FOLIC ACID VITAMIN 5 mg Tablet Oral<br />
884 FOLIC ACID FOLIC ACID 5 mg Tablet Oral<br />
885 FOLICARE FOLIC ACID 2.5mg/5 mls Syrup<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
BEDITA<br />
PHARMACY<br />
FRESENIUS KABI<br />
INDIA PVT.<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
TOBINCO<br />
PHARMACY<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
ASPEE<br />
PHARMACEUT<br />
ICALS LTD.<br />
PHARMANOV<br />
A LTD.<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
UNICHEM<br />
GHANA LTD. 4/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
ALLERGAN<br />
PHARMACEUTICALS BERNSWETT<br />
COMPANY LTD 6/1/2011<br />
ASPEE<br />
PHARMACEUTICALS<br />
LTD.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
ASPEE<br />
PHARMACEUTIC<br />
ALS LTD. 4/1/2013<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 10/4/2013<br />
104<br />
MARCH 1, 2011
886 FOLIGROW<br />
IRON+VITAMIN B<br />
COMPLEX+ZINC Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
887 FOLIKIN FOLIC ACID 5 mg Tablet Oral<br />
888 FORCAN-150 FLUCONAZOLE 150 mg<br />
889<br />
890<br />
FORTIFIED PROCAINE<br />
BENZYLPENICILLIN<br />
FORTIFIED PROCAINE<br />
PENICILLIN INJ.BP<br />
BENZYLPENICILLI<br />
N<br />
SODIUM+PROCAIN<br />
E PENICILLIN 3 MU + 1MU<br />
FORTIFIED<br />
PROCAINE<br />
PENICILLIN 4 mega units<br />
891 FORTUM CEFTAZIDIME 1 g<br />
892 FORTUM CEFTAZIDIME 500 mg<br />
893 FORZID<br />
894 FRAGMIN<br />
GLICLAZIDE+METF<br />
ORMIN<br />
500 mg + 80<br />
mg<br />
DALTEPARIN<br />
SODIUM 2500 iu/Xa<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 10/1/2013<br />
BTP<br />
PHARMACEUT<br />
ICALS CO.<br />
LTD<br />
CHINA<br />
NATIONAL<br />
PHARM.<br />
FOREIGN<br />
TRADE CORP.<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
COLORAMA<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
PFIZER ITALIA<br />
S.r. L.<br />
Yanzhou xier<br />
kangtai<br />
LETAP<br />
pharmaceutical co. PHARMACEUTIC<br />
ltd<br />
ALS LTD 9/1/2013<br />
CHINA NATIONAL<br />
PHARM. FOREIGN<br />
TRADE CORP.<br />
GLAXOSMITHKLINE<br />
S. p. A. (Italy)<br />
GLAXOSMITHKLINE<br />
S. p. A. (Italy)<br />
OSAKA PHARMA<br />
PVT. LTD.<br />
SINOPHARM GH<br />
LTD 6/7/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
TOBINCO<br />
PHARMACY 2/1/2012<br />
VETTER<br />
PHARMAFERTIGUN<br />
G GmbH & CO. GOKALS LTD 4/1/2012<br />
105<br />
MARCH 1, 2011
895 FRAGMIN<br />
896 FREEDOL<br />
DALTEPARIN<br />
SODIUM 5000 iu/ml<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
897 FRELET CLOPIDOGREL 75 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
50 mg + 500<br />
mg Tablet Oral<br />
Tablet film<br />
coated<br />
898 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
899 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
900 FRUSEMIDE FRUSEMIDE 10 mg/ml<br />
Injectable<br />
Solution<br />
901 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
902 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
903 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
904 FRUSEMIDE FRUSEMIDE 10 mg/2ml<br />
Injectable<br />
Solution<br />
PFIZER ITALIA<br />
S.r. L.<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
INNOCIA LIFE<br />
SCIENCES<br />
PVT LTD<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
PFIZER ITALIA S.r.<br />
L. GOKALS LTD 4/1/2012<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
ATOZ<br />
PHARMACEUTICALS<br />
PVT LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
UNICHEM<br />
GHANA LTD. 3/1/2012<br />
106<br />
MARCH 1, 2011
905 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
906 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
907 FRUSEMIDE FRUSEMIDE 40 mg Tablet Oral<br />
908 FUMET<br />
909 FUNBACT-A<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+GENTAMYCIN Multplr<br />
910 FUNGBY FLUCONAZOLE 150 mg<br />
911 FUNGISTOP FLUCONAZOLE 150 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
100 mg/50<br />
mg/5 ml Syrup<br />
PHARMANOV<br />
A LTD.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
Cream<br />
Topical GVS LABS GVS LABS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
912 FUNGOTAB KETOCONAZOLE 200 mg Tablet Oral<br />
913 FURONAT CEFUROXIME 750 mg<br />
Injectable<br />
Powder<br />
914 FURONAT CEFUROXIME 250 mg Tablet Oral<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
PHARMANOVA<br />
LTD. 2/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 4/1/2013<br />
XL LABORATORIES<br />
PVT LTD<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
GR PHARMA<br />
LTD. 8/1/2011<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 8/16/2014<br />
OSON'S CHEMIST<br />
LTD 9/1/2013<br />
OSON'S CHEMIST<br />
LTD 9/1/2013<br />
107<br />
MARCH 1, 2011
915 FYBROGEL ISPAGHULA HUSK 3.5g/sachet<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral Solution<br />
916 GACET Paracetamol 125 mg Suppository<br />
917 GACET Paracetamol 250 mg Suppository<br />
918 GACET-1 gm ACETAMINOPHEN 1 gm Suppository<br />
919 GACET-500 ACETAMINOPHEN 500 mg Suppository<br />
920 GADOVIST GADOBUTROL 1.0 mmol/ml<br />
921 GAMALATE B6<br />
922 GANFORT<br />
Injectable<br />
Solution<br />
MAGNESIUM<br />
GLUTAMATE<br />
HYDROBROMIDE 0,075g Tablet Oral<br />
BIMATOPROST+TI<br />
MOLOL<br />
0.3 mg+5.0<br />
mg Eye drops<br />
923 GANYMOL-1000 PARACETAMOL 1000 mg Suppository<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
BLISS GVS<br />
PHARMA LTD<br />
BLISS GVS<br />
PHARMA LTD<br />
BLISS GVS PHARMA<br />
LTD<br />
BLISS GVS PHARMA<br />
LTD<br />
BAYER -<br />
SCHERING<br />
PHARMA BAYER - SCHERING<br />
AFRICA GMBH PHARMA<br />
FERRER<br />
INTERNACION<br />
AL S.A<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
FERRER<br />
INTERNACIONAL<br />
S.A<br />
ALLERGAN<br />
PHARMACEUTICALS<br />
IRELAND<br />
MERIDIAN<br />
ENTERPRISES<br />
PRIVATE LIMITED<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 10/4/2013<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 4/1/2011<br />
TOBINCO<br />
PHARMACY 4/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 10/1/2013<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 12/1/2012<br />
BERNSWETT<br />
COMPANY LTD 11/1/2013<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 4/1/2011<br />
108<br />
MARCH 1, 2011
924 GANYMOL-125 PARACETAMOL 125 mg Suppository<br />
925 GANYMOL-250 PARACETAMOL 250 mg Suppository<br />
926 GANYMOL-500 PARACETAMOL 500 mg Suppository<br />
927 GASCID<br />
928<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
250mg +<br />
250mg +<br />
25mg<br />
GASEC-20<br />
GASTROCAPS OMEPRAZOLE 20 mg<br />
929 GASTAGIN<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
200 mg/200<br />
mg/25 mg/5<br />
ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
MERIDIAN<br />
ENTERPRISES<br />
PRIVATE LIMITED<br />
MERIDIAN<br />
ENTERPRISES<br />
PRIVATE LIMITED<br />
MERIDIAN<br />
ENTERPRISES<br />
PRIVATE LIMITED<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 4/1/2013<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 4/1/2013<br />
GANYPHARMS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 4/1/2011<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 5/1/2013<br />
Capsule oral<br />
(Hard<br />
gelatin) MEPHA LTD. MEPHA LTD.<br />
Oral<br />
Suspension<br />
MILAN<br />
LABOTRATOR<br />
IES (INDIA)<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
109<br />
MARCH 1, 2011
930 GASTAGIN<br />
931 GASTRACID<br />
932 GASTROGRAFFIN<br />
933 GASTRONE<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
200 mg/200<br />
mg/25 mg<br />
MAGNESIUM<br />
TRISLICATE+MAG<br />
NESIUM<br />
HYDROXIDE+CALC<br />
IUM<br />
CARBONATE+O Multiple<br />
MEGLUMINE<br />
AMIDOTRIZOATE+<br />
SODIUM<br />
AMIDOTRIZOATE<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Chewable<br />
Oral<br />
Suspension<br />
0.1 g/0.66 g/<br />
ml Oral Solution<br />
200 mg/200<br />
mg/25 mg/5<br />
ml<br />
934 GEBECEF CEFTRIAXONE 1000 mg<br />
935 GEBECIP CIPROFLOXACIN<br />
200 mg/100<br />
ml<br />
936 GEBEDICLO DICLOFENAC 50 mg<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
Injectable<br />
Solution<br />
Tablet<br />
Enteric<br />
Coated<br />
MILAN<br />
LABOTRATOR<br />
IES (INDIA)<br />
MEYER<br />
ORGANICS<br />
LTD.<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
MEYER ORGANICS<br />
LTD.<br />
BAYER -<br />
SCHERING<br />
PHARMA BERLIMED, SA<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
ERNEST CHEMISTS<br />
LIMITED<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
GR PHARMA<br />
LTD. 10/1/2013<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 7/1/2011<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
GB PHARMA<br />
(GH) LTD 7/1/2013<br />
GB PHARMA<br />
(GH) LTD 2/1/2013<br />
110<br />
MARCH 1, 2011
937 GEBEDICLO DICLOFENAC 100 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Enteric<br />
Coated<br />
938 GEBEDICLO GEL DICLOFENAC 1 %w/w Gel Topical<br />
939 GEBEDOL<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
940 GEBEFLUC FLUCONAZOLE 150 mg<br />
941 GEBETAX 1 CEFOTAXIME 1 gm<br />
942 GEBETAX 500 CEFOTAXIME 500 mg<br />
943 GEBEVIR ACICLOVIR 200 mg<br />
944 GEBEVIR ACICLOVIR 200mg/5ml<br />
945 GEBEXIME CEFUROXIME 750 mg<br />
500 mg/50<br />
mg Tablet Oral<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Tablet film<br />
coated<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
946 GEBEXIME CEFUROXIME 250 mg Tablet Oral<br />
947<br />
GELOFUSINE 4%<br />
INFUSION GELATIN 4% w/v<br />
Injectable<br />
Solution<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(GH) LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
B. BRAUN<br />
MELSUNGEN<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
GB PHARMA<br />
(GH) LTD 2/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 11/1/2012<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 4/1/2012<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
B. BRAUN<br />
MELSUNGEN<br />
GB PHARMA<br />
(GH) LTD 7/1/2013<br />
GB PHARMA<br />
(GH) LTD 7/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 8/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
NOVARTIS<br />
PHARMA AG 10/1/2013<br />
111<br />
MARCH 1, 2011
948 GELOVITA SYRUP<br />
IRON+MINERALS+V<br />
ITAMINS Multiple Syrup<br />
949 GENTAMICIN GENTAMICIN 3mg/ml<br />
950 GENTAMICIN GENTAMICIN 80 mg<br />
951 GENTAMYCIN GENTAMYCIN 80 mg/2 ml<br />
952 GENTAMYCIN GENTAMICIN 0.3%w/v<br />
953 GENTAMYCIN GENTAMICIN 0.3%w/v<br />
954 GENTI GENTAMICIN 0.3%w/v<br />
955 GENTIAN VIOLET<br />
956 GERICARE<br />
METHYLROSANILI<br />
UM CHLORIDE<br />
(GENTIAN VIOLET) 0.5%w/v<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ear/Eye<br />
Drops<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Ear/Eye<br />
Drops<br />
Ear/Eye<br />
Drops<br />
Ear/Eye<br />
Drops<br />
Topical<br />
solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
OLIVE<br />
HEALTHCARE<br />
ADORE<br />
PHARMACEUT<br />
ICALS PVT.<br />
LTD.<br />
OLIVE<br />
HEALTHCARE<br />
ADORE<br />
PHARMACEUTICALS<br />
PVT. LTD.<br />
GILLIGOLD<br />
PHARMACY LTD 8/1/2011<br />
K . SOMUAH &<br />
SONS<br />
PHARMACY 6/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 3/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
KAMA INDUSTRIES<br />
LTD.<br />
VITABIOTICS<br />
LTD. VITABIOTICS LTD.<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 9/1/2011<br />
112<br />
MARCH 1, 2011
957 GIVITA<br />
MINERALS +<br />
VITAMINS Multiple Syrup<br />
958 GLEVO LEVOFLOXACIN 500 mg Tablet Oral<br />
959 GLIBEMET<br />
GLIBENCLAMIDE+<br />
METFORMIN 5 mg/500 mg Tablet Oral<br />
960 GLIBENC GLIBENCLAMIDE 5 mg Tablet Oral<br />
961 GLIBENCLAMIDE GLIBENCLAMIDE 5 mg Tablet Oral<br />
962 GLIBENCLAMIDE GLIBENCLAMIDE 5 mg Tablet Oral<br />
963 GLIBETICS GLIBENCLAMIDE 5 mg Tablet Oral<br />
964 GLICLAZIDE GLICLAZIDE 80 mg Tablet Oral<br />
965 GLICLAZIDE GLICLAZIDE 80 mg Tablet Oral<br />
966 GLICLAZIDE GLICLAZIDE 80 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
BEDITA<br />
PHARMACY<br />
XL LABORATORIES<br />
PVT LTD<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
MADRAS<br />
PHARMACEUTICALS<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
BEDITA<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 8/1/2013<br />
MINSK<br />
INVESTMENTS<br />
LTD. 6/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 8/1/2012<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
113<br />
MARCH 1, 2011
967<br />
968<br />
969<br />
970<br />
GLO POWER PLUS<br />
SYRUP MULTIVITAMIN Mutiple Syrup<br />
GLUCO-NAF ORANGE<br />
WITH VITAMIN C<br />
GLUCO-NAF<br />
PINEAPPLE WITH<br />
VITAMIN C<br />
GLUCO-NAF WITH<br />
VITAMIN D<br />
GLUCOSE+VITAMI<br />
N C<br />
GLUCOSE+VITAMI<br />
N C<br />
90.4%,<br />
0.165%<br />
90.4%,<br />
0.165%<br />
CALCIUM<br />
GLYCEROPHOSPH<br />
ATE+GLUCOSE+VI<br />
TAMIN D Multiple<br />
971 GLUCOPHAGE METFORMIN 500 mg<br />
972 GLUCOPHAGE METFORMIN 1000 mg<br />
973<br />
GLUCORANGE WITH<br />
VITAMIN C<br />
974 GLUCORYL<br />
GLUCOSE+VITAMI<br />
N C<br />
90%w/w/0.1<br />
75 %w/w<br />
GLUCOSE+VITAMI<br />
N C Multiple<br />
975 GLUCOSE INFUSION GLUCOSE 50 g/l, 5.0g<br />
976<br />
GLUCOSE INSTANT<br />
ENERGY<br />
977 GLUCOVANCE<br />
CALCIUM+GLUCOS<br />
E+VITAMIN D Multiple<br />
GLIBENCLAMIDE+<br />
METFORMIN<br />
5 mg + 500<br />
mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED 8/1/2013<br />
Powder for<br />
Oral Solution DANNEX LTD DANNEX LTD DANNEX LTD 9/18/2013<br />
Powder for<br />
Oral Solution DANNEX LTD DANNEX LTD DANNEX LTD 9/18/2013<br />
Powder for<br />
Oral Solution DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2011<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Powder for<br />
Oral Solution<br />
Powder for<br />
Oral Solution<br />
Injectable<br />
Solution<br />
Powder for<br />
Oral Solution<br />
Tablet film<br />
coated<br />
MERCK SANTE<br />
s.a.s<br />
MERCK SANTE<br />
s.a.s<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
FRESENIUS<br />
KABI INDIA<br />
PVT. LIMITED<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
MERCK SANTE<br />
s.a.s<br />
MERCK SANTE<br />
s.a.s<br />
MERCK SANTE<br />
s.a.s<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
FRESNIUS<br />
MAFATLAL<br />
MEDICALS LTD.<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
MERCK SANTE<br />
s.a.s<br />
BIOFEM GHANA<br />
LTD 6/1/2012<br />
BIOFEM GHANA<br />
LTD 6/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
BIOFEM GHANA<br />
LTD 6/1/2012<br />
114<br />
MARCH 1, 2011
978 GLUCOVANCE<br />
979 GLYCERINE SPIRIT<br />
980<br />
981<br />
GLYCERYL<br />
TRINITRATE<br />
GLYCERYL<br />
TRINITRATE<br />
GLIBENCLAMIDE+<br />
METFORMIN<br />
2.5 mg + 500<br />
mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
GLYCERIN+METHY<br />
LATED SPIRIT BPC Ear Drops<br />
GLYCERYL<br />
TRINITRATE 500 mcg Tablet Oral<br />
GLYCERYL<br />
TRINITRATE 500 mcg Tablet Oral<br />
MERCK SANTE<br />
s.a.s<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
MERCK SANTE<br />
s.a.s<br />
KAMA INDUSTRIES<br />
LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
982 GLYCOBEN GLIBENCLAMIDE 5 mg Tablet Oral GVS LABS GVS LABS<br />
983 GML TONIC<br />
984 GML-APC<br />
985 GO COUGH<br />
986 GOCOLD<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL<br />
150 mg/30<br />
mg/250 mg Tablet Oral<br />
AMMONIUM<br />
CHLORIDE+CHLOR<br />
PHENIRAMINE+DIP<br />
HENHYDRAMINE+S<br />
ODIUM CI Multiple Syrup<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PHENYLEPHRIN<br />
E<br />
500 mg+25<br />
mg+2 mg Tablet Oral<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
BIOFEM GHANA<br />
LTD 6/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 12/1/2012<br />
GEO MEDICORE<br />
LIMITED<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
GEO MEDICORE<br />
LIMITED 12/31/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 8/1/2011<br />
115<br />
MARCH 1, 2011
987 GOGYNAX CLOTRIMAZOLE 100 mg<br />
988 GOLDEN NASAL DROPS<br />
EPHEDRINE+EUCA<br />
LYPTUS+MENTHO<br />
L<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Vaginal<br />
0.75%w/v/0.<br />
2%w/v/0.2%<br />
w/v Nasal Drops<br />
989 GRATONEX MULTIVITAMIN Multiple Tablet Oral<br />
990 GRISEOFULVIN GRISEOFULVIN 500 mg Tablet Oral<br />
991 GRISEOFULVIN GRISEOFULVIN 125 mg Tablet Oral<br />
992 GRISEOFULVIN GRISEOFULVIN 125 mg Tablet Oral<br />
993 GRISERON-500 GRISEOFULVIN 500 mg Tablet Oral<br />
994 GR-METFORMIN METFORMIN 500 mg Tablet Oral<br />
995 GSUNATE 24 KIT<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
153.1 mg/50<br />
mg<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
GOLDEN<br />
TOWER LTD<br />
ABYAK<br />
PHARMA LTD.<br />
GOLDEN TOWER<br />
LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
TROGE<br />
MEDICAL<br />
GMBH MEDOPHARM<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
GR<br />
INDUSTRIES<br />
LTD.<br />
Tablet<br />
Dispersible GVS LABS<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
GR INDUSTRIES<br />
LTD.<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
SOCOMEX<br />
PHARMACY LTD. 11/1/2012<br />
GOLDEN TOWER<br />
LTD 12/31/2011<br />
ABYAK PHARMA<br />
LTD. 12/31/2012<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 11/22/2012<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 2/1/2013<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
GR INDUSTRIES<br />
LTD. 6/1/2013<br />
TOBINCO<br />
PHARMACY 11/1/2011<br />
116<br />
MARCH 1, 2011
996 GV FLUC FLUCONAZOLE 150 mg<br />
997 GV FLUC FLUCONAZOLE 50 mg/5 mls<br />
998 GV FLUC FLUCONAZOLE 50 mg<br />
999 GV FLUC FLUCONAZOLE 200 mg<br />
1000 GVITHER FORTE<br />
BETA-<br />
ARTEMETHER 80 mg/ml<br />
1001 GVITHER-20 ARTEMETHER 20 mg/ml<br />
1002 GVITHER-40 ARTEMETHER 40 mg/ml<br />
1003 GYNAECOSID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Powder for<br />
Oral<br />
Suspension GVS LABS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
BLISS GVS<br />
PHARMA LTD<br />
BLISS GVS<br />
PHARMA LTD<br />
Injectable<br />
Solution GVS LABS<br />
Injectable<br />
Solution GVS LABS<br />
METHYLOESTRADI<br />
OL+METHYLOESTR<br />
ENOLONE 5 mg/0.3 mg Tablet Oral<br />
1004 GYNO DERMOFIX SERTACONAZOLE 500 mg<br />
1005 GYNO-DAKTARIN MICONAZOLE 2% w/w<br />
Tablet<br />
Vaginal<br />
Cream<br />
Vaginal<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)GVS LABS<br />
HEMKISH<br />
PHARMACEUTICALS<br />
& CHEMICALS PVT.<br />
LTD<br />
HEMKISH<br />
PHARMACEUTICALS<br />
& CHEMICALS PVT.<br />
LTD<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
EFROZE<br />
CHEMICAL<br />
INDUSTRIES<br />
(PVT) LTD.<br />
FERRER<br />
INTERNACION<br />
AL S.A<br />
JANSSEN-<br />
CILAG<br />
S.p.A.(ITALY)<br />
KILITCH <strong>DRUG</strong>S<br />
(INDIA) LTD<br />
KILITCH <strong>DRUG</strong>S<br />
(INDIA) LTD<br />
EFROZE CHEMICAL<br />
INDUSTRIES (PVT)<br />
LTD.<br />
FERRER<br />
INTERNACIONAL<br />
S.A<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
DOVE<br />
PHARMACY<br />
LIMITED 2/1/2013<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 12/1/2012<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
117<br />
MARCH 1, 2011
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
1006 GYNOFIT Gel Topical TENTAN AG MEDENA AG<br />
1007 GYNOFIT LACTIC LACTIC ACID Gel Topical TENTAN AG MEDENA AG<br />
1008 GYNO-MYCOLEX MICONAZOLE 400 mg<br />
1009 GYNO-PEVARYL ECONAZOLE 150 mg<br />
1010 GYNO-TRAVOGEN ISOCONAZOLE 600 mg<br />
1011 GYPRONE PLUS<br />
1012 GYPRONE PLUS<br />
1013 HAEMACCEL<br />
1014 HAEMO FORTE<br />
1015 HAEMOGLOBIN<br />
Ovule<br />
Vaginal<br />
Ovule<br />
Vaginal<br />
Ovule<br />
Vaginal<br />
CYPROHEPTADINE<br />
+MINERALS+VITAM<br />
INS Multiple Tablet Oral<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
POLYGELINE<br />
SOLUTION 3.5 %w/v<br />
Injectable<br />
Solution<br />
FOLIC<br />
ACID+IRON+MULTI<br />
VITAMINS Multiple Syrup<br />
HAEMOGLOBIN+VI<br />
TAMIN B12<br />
0.2 g/20<br />
mcg/5ml Syrup<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
JANSSEN-<br />
CILAG<br />
S.p.A.(ITALY)<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
PIRAMAL<br />
HEALTHCARE<br />
LIMITED (UK)<br />
ROYAL<br />
MULTIPURPO<br />
SE GROUP<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
PSA CHEMICALS &<br />
PHARMACEUTICALS<br />
PVT. LTD<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
BAYER - SCHERING<br />
PHARMA<br />
SOCOMED PHARMA<br />
PVT LTD<br />
SOCOMED PHARMA<br />
PVT LTD<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED (INDIA)<br />
SEMOS<br />
PHARMACEUTICALS<br />
(PVT) LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
SWISSGHA<br />
PHARMA LTD 10/1/2011<br />
SWISSGHA<br />
PHARMA LTD 10/1/2011<br />
UNICHEM<br />
GHANA LTD. 7/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
BEDITA<br />
PHARMACY 3/1/2013<br />
BEDITA<br />
PHARMACY 7/1/2013<br />
PHARMANOVA<br />
LTD. 8/1/2013<br />
HOUSE OF<br />
<strong>DRUG</strong>S AND<br />
TRADING ENT.<br />
LTD. 9/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
118<br />
MARCH 1, 2011
1016<br />
HAEMOGLOBIN WITH<br />
VIT B12<br />
1017 HAEMOVITEX FORTE<br />
HAEMOGLOBIN+VI<br />
TAMIN B12<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
0.21 g/15<br />
mcg/5ml Syrup<br />
FOLIC<br />
ACID+IRON+VITAM<br />
IN B12 Multiple Syrup<br />
1018 HALOPERIDOL HALOPERIDOL 10 mg Tablet Oral<br />
1019 HALOPERIDOL HALOPERIDOL 5 mg/ml<br />
1020 HALOTHANE HALOTHANE 100 %<br />
1021 HALOTHANE HALOTHANE 100% w/v<br />
1022 HAVRIX HEPATITIS A 1440<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Inhalational<br />
solution<br />
Injectable<br />
Suspension<br />
1023 HAYZINE CETIRIZINE 10 mg Caplets oral<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
GRACURE<br />
PHARMACEUT<br />
ICALS LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 1/4/2013<br />
SECOM<br />
PHARMACY LTD 4/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED<br />
(INDIA)<br />
GLAXOWELLC<br />
OME SOUTH<br />
AFRICA (PTY)<br />
LTD.<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED (INDIA)<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED (INDIA)<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
ZETA<br />
PHARMACY LTD 12/31/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 8/1/2011<br />
119<br />
MARCH 1, 2011
1024 HB TONE<br />
1025 HBGLOW<br />
1026 HBGLOW<br />
1027<br />
HEATOL CHINESE<br />
BALM<br />
CALCIUM+FOLIC 75 mg + 0.25<br />
ACID+IRON+VITAM mg + 129.5<br />
IN B12<br />
mg + 1.25 mg Syrup<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple<br />
MENTHOL+PEPPER<br />
MINT+OTHERS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Cream<br />
Topical<br />
1028 HELMAZOLE ALBENDAZOLE 200 mg Tablet Oral<br />
1029 HELMAZOLE ALBENDAZOLE 200 mg/5 mls<br />
1030 HEPARIN SODIUM HEPARIN SODIUM 500 iu/5 ml<br />
1031<br />
HEPARIN SODIUM-<br />
FRESENIUS HEPARIN SODIUM 5000 iu/ml<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
Injectable<br />
Suspension<br />
GEO<br />
MEDICORE<br />
LIMITED GEO PHARMACY 12/1/2012<br />
UNIVERSAL<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
UNIVERSAL<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
PZ CUSSONS<br />
GH. LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
UNIVERSAL<br />
PHARMACEUTICALS<br />
LIMITED<br />
UNIVERSAL<br />
PHARMACEUTICALS<br />
LIMITED<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)PZ<br />
CUSSONS GH. LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED HIPLUS,S.A<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
120<br />
MARCH 1, 2011
1032 HEPTAMIN<br />
1033 HEPTAVIT<br />
1034 HEPTOLIF<br />
CYPROHEPTADINE<br />
+MINERALS+VITAM<br />
INS Multiple Syrup<br />
AMINO<br />
ACIDS+CYPROHEP<br />
TADINE+VITAMINS Multiple Syrup<br />
CYPROHEPTADINE<br />
+MINERALS+PROT<br />
EIN+VITAMINS Multiple Syrup<br />
1035 HERCEPTIN TRASTUZUMAB 440.00 mg<br />
1036 HESTAR<br />
HYDROXYETHYL<br />
STARCH 200 mg<br />
1037 HIDRODROID Multiple<br />
1038 HISTARINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
Cream<br />
Topical<br />
CHLORPHENIRAMI<br />
NE 4 mg Tablet Oral<br />
1039 HISTAZINE CETIRIZINE 10 mg Tablet Oral<br />
1040 HUMALOG HUMAN INSULIN 100 IU<br />
Injectable<br />
Suspension<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
F. HOFFMAN-<br />
LA ROCHE<br />
INC(USA)<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ELI LILLY AND<br />
(suisse) SA (<br />
Kenya)<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 2/1/2013<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 2/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 9/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
LILLY FRANCE S.<br />
A. REISS & CO. 7/1/2012<br />
121<br />
MARCH 1, 2011
1041 HUMULIN 70/30 HUMAN INSULIN<br />
1042 HUMULIN N HUMAN INSULIN 100 iu/ml<br />
1043 HUMULIN R HUMAN INSULIN 100 units<br />
1044 HYDRALAZINE HYDRALAZINE 25 mg<br />
1045 HYDRALAZINE HYDRALAZINE 25 mg<br />
1046<br />
HYDROCHLORTHIAZID<br />
E<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Suspension<br />
Injectable<br />
Suspension<br />
Injectable<br />
Suspension<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
HYDROCHLORTHI<br />
AZIDE 25 mg Tablet Oral<br />
1047 HYDROCORTISONE HYDROCORTISONE 1% w/w<br />
1048 HYDROCORTISONE HYDROCORTISONE 100 mg<br />
1049 HYDROCORTISONE HYDROCORTISONE 1% w/w<br />
Cream<br />
Topical<br />
Injectable<br />
Solution<br />
Cream<br />
Topical<br />
LILLY EGYPT<br />
S.A.E. & CO.<br />
LILLY EGYPT<br />
S.A.E. & CO.<br />
LILLY EGYPT<br />
S.A.E. & CO.<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LILLY EGYPT S.A.E.<br />
& CO. REISS & CO. 12/1/2012<br />
LILLY EGYPT S.A.E.<br />
& CO. REISS & CO. 12/1/2012<br />
LILLY EGYPT S.A.E.<br />
& CO. REISS & CO. 12/1/2012<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 10/3/2013<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
122<br />
MARCH 1, 2011
1050<br />
1051<br />
HYDROCORTISONE<br />
SODIUM HYDROCORTISONE 100 mg<br />
HYDROCORTISONE<br />
SODIUM SUCCINATE HYDROCORTISONE 100 mg/vial<br />
1052 HYOMAX<br />
1053<br />
HYOSCINE<br />
BUTYLBROMIDE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
HYOSCINE<br />
BUTYLBROMIDE 10 mg Tablet Oral<br />
HYOSCINE<br />
BUTYLBROMIDE 20 mg<br />
Injectable<br />
Powder<br />
1054 HYPARIL LISINOPRIL 5 mg Tablet Oral<br />
1055 HYPARIL LISINOPRIL 10 mg Tablet Oral<br />
1056 HYTRIN TERAZOSIN 2 mg Tablet Oral<br />
1057 HYTRIN TERAZOSIN 5 mg Tablet Oral<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
MONA<br />
PHARMA LTD<br />
AMBICA<br />
PHARMA<br />
SALES<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT<br />
LTD<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT<br />
LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
VAST FAVOUR<br />
INTERNATIONAL<br />
LTD<br />
PRIME<br />
PHARMACEUTICALS<br />
LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
AKUM <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
LTD<br />
AKUM <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
LTD<br />
OSON'S CHEMIST<br />
LTD 4/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
MONA PHARMA<br />
LTD 11/1/2013<br />
SPINTEX<br />
CHEMIST LTD 12/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
123<br />
MARCH 1, 2011
1058 IBANATE 25/67.5<br />
1059 IBANATE 50/135<br />
1060<br />
1061<br />
IBANTE ADULT<br />
100/270<br />
IBANTE CHILD<br />
100/270<br />
1062 IBATHER<br />
1063 IBROPACK<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
67.5 mg + 25<br />
mg Tablet Oral<br />
135 mg + 50<br />
mg Tablet Oral<br />
270 mg + 100<br />
mg Tablet Oral<br />
270 mg + 100<br />
mg Tablet Oral<br />
20 mg + 120<br />
mg Tablet Oral<br />
1064 IBUFEN IBUPROFEN 400 mg Tablet Oral<br />
1065 IBUP<br />
1066 IBUP-PLUS<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
30 mg/200<br />
mg/250 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
30mg +<br />
400mg +<br />
500mg Tablet Oral<br />
1067 IBUPROFEN IBUPROFEN 200 mg Tablet Oral<br />
1068 IBUPROFEN IBUPROFEN 400 mg Tablet Oral<br />
LUPIN<br />
LIMITED LUPIN LIMITED 9/1/2012<br />
LUPIN<br />
LIMITED LUPIN LIMITED<br />
LUPIN<br />
LIMITED LUPIN LIMITED<br />
RYZENPHARMA<br />
LIMITED 9/1/2012<br />
RYZENPHARMA<br />
LIMITED 9/1/2012<br />
LUPIN<br />
LIMITED LUPIN LIMITED 9/1/2012<br />
LUPIN<br />
LIMITED LUPIN LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
RYZENPHARMA<br />
LIMITED 9/1/2012<br />
BONS<br />
PHARMACY LTD 7/1/2013<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 12/20/2013<br />
GEO MEDICORE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED 8/1/2013<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
124<br />
MARCH 1, 2011
1069 IBUPROFEN IBUPROFEN 400 mg Tablet Oral<br />
1070 IBUPROFEN IBUPROFEN 100 mg<br />
1071 IBURICH<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
30 mg+ 200<br />
mg+ 325 mg<br />
1072 IBURON IBUPROFEN 100 mg/5 ml<br />
1073 ICHTAMMOL ICHTAMMOL 100 %<br />
1074 IDEOS<br />
CALCIUM<br />
CARBONATE +<br />
CHOLECALCIFERO<br />
L<br />
500 mg + 400<br />
IU<br />
1075 IDICEF CEFIXIME 100 mg/5 mls<br />
1076 IMIDIL TOPICAL CLOTRIMAZOLE 1 %w/w<br />
1077 IMIDIL VAGINAL CLOTRIMAZOLE 100 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Oral<br />
Suspension<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 12/31/2012<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/20/2015<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 12/31/2012<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2012<br />
Tablet<br />
Chewable<br />
Oral<br />
Suspension<br />
Cream<br />
Topical<br />
Tablet<br />
Vaginal<br />
LABORATOIRE<br />
INNOTECH<br />
INTERNATION<br />
AL<br />
GB PHARMA<br />
(uk) LTD.<br />
LYKA LABS.<br />
LIMITED<br />
LYKA LABS.<br />
LIMITED<br />
INNOTHERA<br />
CHOUZY<br />
(INNOTHERA<br />
GROUP)<br />
MISSION VIVACARE<br />
LIMITED<br />
LYKA LABS.<br />
LIMITED<br />
LYKA LABS.<br />
LIMITED<br />
GOKALS-<br />
LABOREX LTD 9/9/2012<br />
GB PHARMA<br />
(GH) LTD 11/1/2012<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 5/1/2012<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 6/1/2012<br />
125<br />
MARCH 1, 2011
1078 IMIPRAMINE IMIPRAMINE 25 mg Tablet Oral<br />
1079 IMODIUM LOPERAMIDE 2 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1080 IMPLANON ETONORGESTREL 68 mg Implant<br />
1081 INDOMETHACIN INDOMETHACIN 25 mg<br />
1082 INDOMETHACIN INDOMETHACIN 25 mg<br />
1083 INDOMETHACIN INDOMETHACIN 25 mg<br />
1084 INFACOL SIMETHICONE 40 mg/ml Syrup<br />
1085 INHALRON<br />
CAMPHOR+CHLOR<br />
THYMOL+EUCALY<br />
PTUS+MENTHOL+<br />
TERPINEOL Multiple<br />
1086 INOSERT-100 SERTRALINE 100 mg<br />
1087 INOSERT-50 SERTRALINE 50 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule for<br />
Inhalation<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
BEDITA<br />
PHARMACY<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
N.V.<br />
ORGANON N.V. ORGANON<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
GEO MEDICORE<br />
LIMITED<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
GEO MEDICORE<br />
LIMITED 12/31/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 6/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 7/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 7/1/2011<br />
126<br />
MARCH 1, 2011
1088<br />
INSULATARD HM<br />
Injectable<br />
PENFILL HUMAN INSULIN 100 iu/1.5 ml Suspension<br />
1089 INSUMAN BASAL INSULIN 100 IU/ml<br />
1090 INTAVITA<br />
1091 INTRAMAG<br />
1092 INTRON A 18<br />
1093 INTRON A 25<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
MAGNESIUM<br />
SULFATE 0.5 g/ml<br />
INTERFERON ALFA-<br />
2B 18 MIU/ vial<br />
INTERFERON ALFA-<br />
2B 25 MIU/vial<br />
1094 INVIRASE SAQUINAVIR 500 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1095 IODINE TINCTURE IODINE 2.5 % + 2.5% Mixtures<br />
ALDAPH SPA<br />
(NOVO<br />
NORDISK<br />
ALGERIA)<br />
SANOFI<br />
AVENTIS<br />
NIGERIA<br />
LIMITED<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
INTRAVENOU<br />
S INFUSIONS<br />
LTD<br />
SCHERING-<br />
PLOUGH<br />
(PTY)<br />
LIMITED<br />
SCHERING-<br />
PLOUGH<br />
(PTY)<br />
LIMITED<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
ALDAPH SPA (NOVO<br />
NORDISK<br />
ALGERIA) DANAFCO LTD 4/1/2011<br />
SANOFI-AVENTIS<br />
DEUTSCHLAND<br />
GmbH<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
INTRAVENOUS<br />
INFUSIONS LTD<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND)<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND)<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
KAMA INDUSTRIES<br />
LTD.<br />
GOKALS-<br />
LABOREX LTD 7/1/2013<br />
GR PHARMA<br />
LTD. 7/1/2013<br />
INTRAVENOUS<br />
INFUSIONS LTD 4/1/2011<br />
PRIME HEALTH<br />
SERVICES<br />
LIMITED 10/1/2013<br />
PRIME HEALTH<br />
SERVICES<br />
LIMITED 10/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
127<br />
MARCH 1, 2011
1096 IOPAMIRO 300 IOPAMIDOL 30g/100 mls<br />
1097 IOPAMIRO 370 IOPAMIDOL 37g/100 ml<br />
1098 IRON DEXTRAN IRON DEXTRAN 50 mg<br />
1099 ISOKIN<br />
1100 ISOKIN<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM 240 mg/5ml<br />
1101 ISOPRENALINE ISOPRENALINE 2 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
400 mg/80<br />
mg Tablet Oral<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
1102 ISOPTO CARPINE PILOCARPINE 4% w/v Eye drops<br />
1103<br />
ISOSORBIDE<br />
DINITRATE ISOSORBIDE 10 mg Tablet Oral<br />
1104 ISTIN<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
BRACCO<br />
S.P.A BRACCO S.P.A<br />
BRACCO<br />
S.P.A BRACCO S.P.A<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
ALCON<br />
PHARMACEUTICALS<br />
G. D. COOPER &<br />
CO. LIMITED<br />
CYNDEX<br />
PHARMACY 12/31/2011<br />
CYNDEX<br />
PHARMACY 12/31/2011<br />
OSON'S CHEMIST<br />
LTD 4/1/2013<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 3/24/2014<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
128<br />
MARCH 1, 2011
1105 ISTIN<br />
1106 ITCHGUARD<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
CLOTRIMAZOLE+I<br />
TCHAMMOL+ZINC<br />
OXIDE+BORIC<br />
ACID+OTHERS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
PARAS<br />
PHARMACEUT<br />
ICALS LTD<br />
PARAS<br />
PHARMACEUTICALS<br />
LTD<br />
1107 JADELLE LEVONORGESTREL 75 mg Implant LEIRAS OY LEIRAS OY<br />
1108 JEDITONE<br />
1109 JET 2 INHALER<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Syrup<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
1110 KABIVEN Multiple<br />
1111 KAMAGYL METRONIDAZOLE 125 mg/ 5mls<br />
1112 KAMALOPE LOPERAMIDE 2 mg<br />
Inhalational<br />
solution<br />
Injectable<br />
emulsion<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1113 KAMAMET METFORMIN 500 mg Tablet Oral<br />
TOBINCO<br />
PHARMACY<br />
CEDAR POINT<br />
CHEMIST<br />
FRESENIUS<br />
KABI (PTY)<br />
LTD (SOUTH<br />
AFRICA)<br />
GRACURE<br />
PHARMACEUT<br />
ICALS LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
KONGHUAM OHSOT<br />
STORE<br />
FRESENIUS KABI<br />
AUSTRIA<br />
GMBHFRESENIUS<br />
KABI AB (SWEDEN)<br />
GRACURE<br />
PHARMACEUTICALS<br />
LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
CEDAR POINT<br />
CHEMIST 6/1/2013<br />
HILLS<br />
PHARMACY 6/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
129<br />
MARCH 1, 2011
1114 KAMATONE<br />
1115 KAMATONE<br />
1116 KAMATRIM<br />
1117 KAMCLOX<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC multiple Syrup<br />
MULTIVITAMIN+MI<br />
NERALS multiple<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
AMPICILLIN+CLOX<br />
ACILLIN<br />
200 mg/40<br />
mg/5 mls<br />
125mg/125<br />
mg/5mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
1118 KAMOL PARACETAMOL 120 mg/5 mls Syrup<br />
1119 KAMOXYL AMOXICILLIN 125 mg/5 mls<br />
Powder for<br />
Oral<br />
Suspension<br />
1120 KAMPRED PREDNISOLONE 0.10%w/v Eye drops<br />
1121 KAMPRED PREDNISOLONE 0.52%w/v Eye drops<br />
1122 KAMPRED N<br />
NEOMYCIN+PREDN<br />
ISOLONE<br />
0.5%w/w+0.5<br />
2%w/v Eye drops<br />
1123 KARDAK 20 SIMVASTATIN 20 mg Tablet Oral<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
SANKA (UK)<br />
LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
GRACURE<br />
PHARMACEUT<br />
ICALS LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
KAMA INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
SHREECHEM<br />
LABORATORIES PASS PHARMACY 9/1/2012<br />
KAMA INDUSTRIES<br />
LTD.<br />
GRACURE<br />
PHARMACEUTICALS<br />
LTD<br />
KAMA INDUSTRIES<br />
LTD.<br />
KAMA INDUSTRIES<br />
LTD.<br />
KAMA INDUSTRIES<br />
LTD.<br />
AUROBINDO<br />
PHARMA LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 8/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
130<br />
MARCH 1, 2011
1124 KARDAK 40 SIMVASTATIN 40 mg Tablet Oral<br />
1125 KEFROX CEFUROXIME 125 mg/5 mls<br />
1126 KENARTE ARTEMETHER 80 mg<br />
1127 KENTRIX CEFTRIAXONE 1 g<br />
1128 KERZOL ALBENDAZOLE 200 mg/5 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Oral<br />
Suspension<br />
1129 KERZOL ALBENDAZOLE 400 mg Tablet Oral<br />
1130 KETAMINE KETAMINE 50 mg/ml<br />
1131 KETAMINE KETAMINE 50 mg/ml<br />
1132 KETAMIN-FRESENIUS KETAMINE 50 mg/ ml<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
INDUSKEN<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
INDUSKEN<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
AUROBINDO<br />
PHARMA LIMITED<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
SAMRUDH<br />
PHARMACEUTICALS<br />
PVT. LTD.<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
UNICHEM<br />
GHANA LTD. 10/1/2011<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 11/1/2012<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 11/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
131<br />
MARCH 1, 2011
1133 KETAZOL KETOCONAZOLE 2 %w/v<br />
1134 KETOCONAZOLE KETOCONAZOLE 2 %w/v<br />
1135 KETONAL DUO KETOPROFEN 150 mg<br />
1136 KETOPLUS SHAMPOO<br />
KETOCONAZOLE+<br />
ZINC PYRITHIONE<br />
2%w/w/1%w/<br />
w<br />
1137 KETOPROFEN KETOPROFEN 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
Lotion<br />
Topical<br />
Lotion<br />
Capsule<br />
Extended<br />
Release<br />
Topical<br />
Lotion<br />
Capsule<br />
Delayed<br />
Release<br />
1138 KETOPROFEN KETOPROFEN 5w/w Gel Topical<br />
1139 KIBUFEN IBUPROFEN 100 mg/5 mls<br />
1140 KIDICARE<br />
Oral<br />
Suspension<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
SHALINA<br />
LABORATORIE GOPALDAS VISRAM<br />
S PVT. LTD. & CO. LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
MEYER<br />
ORGANICS<br />
LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
KAMA INDUSTRIES<br />
LTD.<br />
MEYER ORGANICS<br />
LTD.<br />
SOCOMEX<br />
PHARMACY LTD. 3/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
SUPRA PHARMA<br />
LIMITED 5/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
GR PHARMA<br />
LTD. 7/1/2011<br />
132<br />
MARCH 1, 2011
1141 KIDIMIN<br />
1142 KINAGEN<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
PROMETHAZINE<br />
HYDROCHLORIDE 5 mg/5mls Syrup<br />
1143 KINAMOX AMOXICILLIN 500 mg<br />
1144 KINAMOX AMOXICILLIN 125 mg/5mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Powder for<br />
Oral<br />
Suspension<br />
1145 KINAMYCIN CLINDAMYCIN 150 mg Caplets oral<br />
1146 KINAMYCIN CLINDAMYCIN 300 mg Caplets oral<br />
1147 KLIRE<br />
1148 KLIRE ANTACID<br />
1149 KLIRE-4 WAY COUGH<br />
PARACETAMOL+DI<br />
CLOFENAC<br />
SODIUM<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS<br />
AMMONIUM<br />
CHLORIDE+BROM<br />
HEXINE+DEXTROM<br />
ETHORPHAN+MEN<br />
THOL<br />
650 mg/50<br />
mg Tablet Oral<br />
250 mg/250<br />
mg/50 mg/10 Oral<br />
mg<br />
Suspension<br />
10 mg/100<br />
mg/8 mg/5<br />
mg Syrup<br />
SENES<br />
PHARMA CO.<br />
LTD<br />
PREGEN EXPORTS<br />
(INDIA)<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
ROKMER<br />
PHARMA<br />
LIMITED<br />
ROKMER<br />
PHARMA<br />
LIMITED<br />
ROKMER<br />
PHARMA<br />
LIMITED<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
SENES PHARMA<br />
CO. LTD 12/31/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
KINAPHARMA<br />
LTD 7/1/2011<br />
KINAPHARMA<br />
LTD 7/1/2011<br />
ROKMER<br />
PHARMA<br />
LIMITED 4/1/2013<br />
ROKMER<br />
PHARMA<br />
LIMITED 4/1/2011<br />
ROKMER<br />
PHARMA<br />
LIMITED 4/1/2011<br />
133<br />
MARCH 1, 2011
1150 KNZ-200 KETOCONAZOLE 200 mg Tablet Oral<br />
1151 KOACT<br />
1152 KOACT-1000<br />
1153 KOFFEX A DRY COUGH<br />
1154 KOFFEX COUGH<br />
1155 KOFLYN JUNIOR<br />
1156 KOLRON<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 625 Tablet Oral<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
875 mg/125<br />
mg Tablet Oral<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 9/1/2013<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 7/1/2013<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 7/1/2013<br />
DEXTROMETHORP 10 mg + 14<br />
HAN+DIPHENHYDR mg +1.1<br />
AMINE+MENTHOL mg/5ml Syrup DANNEX LTD DANNEX LTD DANNEX LTD 11/30/2014<br />
DIPHENHYDRAMIN<br />
E+SODIUM<br />
CITRATE<br />
7 mg/28.50<br />
mg Syrup DANNEX LTD DANNEX LTD DANNEX LTD 4/1/2013<br />
DIPHENHYDRAMIN<br />
E+SODIUM<br />
CITRATE Multiple Syrup<br />
CHLORPHENIRAMI<br />
NE+DEXTROMETH<br />
ORPHAN+PHENYL<br />
PROPANOLAMINE<br />
2 mg/10<br />
mg/12.50 mg<br />
1157 KONAFLOX TM OFLOXACIN 400 mg<br />
Capsule oral<br />
(Softgel)<br />
Tablet film<br />
coated<br />
1158 KWAI Multiple Tablet Oral<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
ULTRAS<br />
PHARMACEUT<br />
ICALS INC<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
ERNEST CHEMISTS<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
LABORATORIOS<br />
KENER SA<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ULTRAS<br />
PHARMACEUTIC<br />
ALS INC 3/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
134<br />
MARCH 1, 2011
1159 KYTRIL GRANISETRON 1 mg Tablet Oral<br />
1160 KYTRIL GRANISETRON 1 mg/ml<br />
1161 LABETALOL LABETALOL 5 mg/ ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
1162 LACTODEL BROMOCRIPTINE 2.50 mg Tablet Oral<br />
1163 LACTULOSE SOLUTION LACTULOSE BP Oral Solution<br />
1164 LACTULOSE SOLUTION LACTULOSE BP Oral Solution<br />
1165 LAMISIL TERBINAFINE 250 mg Tablet Oral<br />
1166 LAMISIL TERBINAFINE 1 %w/w<br />
Cream<br />
Topical<br />
1167 LAMIVUDINE LAMIVUDINE 150 mg Tablet Oral<br />
1168 LAMIVUDINE LAMIVUDINE 300 mg Tablet Oral<br />
F. HOFFMAN-<br />
LA ROCHE<br />
INC(USA)<br />
F. HOFFMAN-<br />
LA ROCHE<br />
INC(USA)<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
AMOUN<br />
PHARMACEUT<br />
ICALS<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
NOVARTIS<br />
PHARMA AG<br />
BETA<br />
HEALTHCARE<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
F. HOFFMAN-LA<br />
ROCHE INC(USA)<br />
F. HOFFMAN-LA<br />
ROCHE INC(USA)<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 3/17/2014<br />
AMOUN<br />
PHARMACEUTICALS REHA MEDICAL<br />
SUPPLY 2/1/2013<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD (UK)<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
STRIDES ARCOLAB<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
135<br />
MARCH 1, 2011
1169 LAMIVUDINE LAMIVUDINE 150 mg<br />
1170<br />
1171<br />
1172<br />
LAMIVUDINE&STAVUDI<br />
NE<br />
LAMIVUDINE&ZIDOVU<br />
DINE<br />
LAMIVUDINE+ZIDOVUD<br />
INE<br />
1173 LAMONTE-BG<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
LAMIVUDINE+STA<br />
Tablet film<br />
VUDINE 150mg/30 mg coated<br />
LAMIVUDINE+ZIDO<br />
VUDINE<br />
LAMIVUDINE+ZIDO<br />
VUDINE<br />
150mg/300<br />
mg<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+GENTAMYCIN MULTIPLE<br />
Tablet film<br />
coated<br />
150 mg/300<br />
mg Tablet Oral<br />
Cream<br />
Topical<br />
1174 LANDER POLAR ICE MENTHOL 2.5%w/w Gel Topical<br />
1175 LANSOPRAZOLE LANSOPRAZOLE 30 mg<br />
1176 LANSOPRAZOLE LANSOPRAZOLE 15 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
GENO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
UNIROB<br />
PHARMACEUT<br />
ICALS LTD<br />
(Universal<br />
Hospitals<br />
Group Ltd)<br />
STRIDES ARCOLAB<br />
LIMITED<br />
GENO<br />
PHARMACEUTICALS<br />
LIMITED<br />
OLEANDER BRANDS<br />
INTERNATIONAL<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
HILLS<br />
PHARMACY 6/1/2011<br />
UNIROB<br />
PHARMACEUTIC<br />
ALS LTD<br />
(Universal<br />
Hospitals Group<br />
Ltd) 2/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
136<br />
MARCH 1, 2011
1177 LANTUS INSULIN HUMAN INSULIN 100 iu/ml<br />
1178 LARGESIC<br />
1179 LEKADOL PLUS C<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
PARACETAMOL+VI<br />
TAMIN C<br />
50 mg + 500<br />
mg<br />
500 mg/300<br />
mg<br />
1180 LEMFORMIN 500 METFORMIN 500 mg<br />
1181 LEMSIP Multiple<br />
1182 LEO RUB<br />
1183 LEOPARD BAUME<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE Multiple<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Suspension<br />
Tablet film<br />
coated<br />
Granules For<br />
Oral Use<br />
Tablet film<br />
coated<br />
Cream<br />
Topical<br />
Ointment<br />
Topical<br />
1184 LEPONEX CLOZAPINE 100 mg Tablet Oral<br />
AVENTIS<br />
PHARMA<br />
DEUTSCHLAN<br />
D<br />
AVENTIS PHARMA<br />
DEUTSCHLAND GOKALS LTD 10/1/2013<br />
LARK LARK<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
HANS-E.<br />
LEMBCKE<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
GB PHARMA<br />
(uk) LTD.<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
MISSION VIVACARE<br />
LIMITED<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
NOVARTIS<br />
FARMA SPA<br />
(ITALY)<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD (UK)<br />
BIG MARON<br />
PHARMACY 9/1/2012<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
FRED'S<br />
PHARMACY 9/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
GB PHARMA<br />
(GH) LTD 10/1/2012<br />
SOCOMEX<br />
PHARMACY LTD. 11/1/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
137<br />
MARCH 1, 2011
1185 LEPONEX CLOZAPINE 25 mg Tablet Oral<br />
1186 LESCOL FLUVASTATIN 20 mg<br />
1187 LESCOL XL FLUVASTATIN 40 mg<br />
1188 LESCOL XL FLUVASTATIN 80 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1189 LETAFEN IBUPROFEN 400 mg Tablet Oral<br />
1190 LETAFEN IBUPROFEN 200 mg Tablet Oral<br />
1191<br />
LETALIN<br />
EXPECTORANT<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE+ME<br />
NTHOL+SODIUM<br />
CITRATE Multiple Syrup<br />
1192 LETAMOL PARACETAMOL 500 mg Tablet Oral<br />
1193 LETAMOL ELIXIR PARACETAMOL 120 mg/5 ml Syrup<br />
NOVARTIS<br />
FARMA SPA<br />
(ITALY)<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
NOVARTIS<br />
PHARMACEUTICALS<br />
LTD (UK)<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
138<br />
MARCH 1, 2011
1194 LETAMOX MEBENDAZOLE 100 mg Tablet Oral<br />
1195 LETAMOX MEBENDAZOLE 100 mg/5 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
1196 LETAPLEX-B MULTIVITAMIN Multiple Syrup<br />
1197 LETAQUINE CHLOROQUINE 80 mg/ 5ml Syrup<br />
1198 LETAVIN GRISEOFULVIN 125 mg Tablet Oral<br />
1199 LETAVIT MULTIVITAMIN Multiple<br />
Tablet film<br />
coated<br />
1200 LETAVIT MULTIVITAMIN Multiple Syrup<br />
1201 LETAVIT PLUS MULTIVITAMIN Multiple Syrup<br />
1202 LEVATAX LEVAMISOLE 40 mg<br />
Tablet film<br />
coated<br />
1203 LEVITRA VARDENAFIL 5 mg Tablet Oral<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
GEO MEDICORE<br />
LIMITED<br />
BAYER-SCHERING<br />
HEALTHCARE AG<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 6/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
GEO MEDICORE<br />
LIMITED 8/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
139<br />
MARCH 1, 2011
1204 LEVITRA VARDENAFIL 10 mg Tablet Oral<br />
1205 LEVITRA VARDENAFIL 20 mg Tablet Oral<br />
1206 LEVOTHYROXINE LEVOTHYROXINE 50 mcg Tablet Oral<br />
1207 LEVOTHYROXINE LEVOTHYROXINE 50 mcg Tablet Oral<br />
1208 LEVOTHYROXINE LEVOTHYROXINE 100 mcg Tablet Oral<br />
1209 LEVOTHYROXINE LEVOTHYROXINE 100 mcg Tablet Oral<br />
1210 LEXAGLOBIN<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg/500<br />
mcg/5mls Syrup<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
BAYER-SCHERING<br />
HEALTHCARE AG<br />
BAYER-SCHERING<br />
HEALTHCARE AG<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
AGLOWMED<br />
LIMITED<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 5/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 4/1/2012<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
140<br />
MARCH 1, 2011
1211 LEXAGLOBIN<br />
1212 LEXCOLD<br />
1213 LEXOCAP<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO 125 mg/ 12.5<br />
L+PHENYLPROPAN mg/2 mg/5<br />
OLAMINE mls Syrup<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
30 mg+200<br />
mg+325 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1214 LEXOTANIL BROMAZEPAM 1.5 mg Tablet Oral<br />
1215 LEXSPORIN<br />
1216 LG-AMLODAC<br />
1217 LG-AMLODAC<br />
BACITRACIN<br />
ZINC+NEOMYCIN+<br />
POLYMYXIN B Multiple<br />
Ointment<br />
Topical<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
UNICHEM<br />
INDUSTRIES<br />
LTD<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
CADILA<br />
(HEALTHCAR<br />
E)<br />
PHARMACEUT<br />
ICALS LTD.<br />
CADILA<br />
(HEALTHCAR<br />
E)<br />
PHARMACEUT<br />
ICALS LTD.<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
UNICHEM<br />
INDUSTRIES LTD<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
AUROCHEM<br />
PHARMACEUTICALS<br />
PVT LTD.<br />
CADILA<br />
(HEALTHCARE)<br />
PHARMACEUTICALS<br />
LTD.<br />
CADILA<br />
(HEALTHCARE)<br />
PHARMACEUTICALS<br />
LTD.<br />
UNICHEM<br />
GHANA LTD. 12/1/2012<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
UNICHEM<br />
GHANA LTD. 6/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 8/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 8/1/2011<br />
141<br />
MARCH 1, 2011
1218 LG-GLIZONE PIOGLITAZONE 15 mg Tablet Oral<br />
1219 LG-GLIZONE PIOGLITAZONE 30 mg Tablet Oral<br />
1220 LIBRUKIN<br />
CHLORDIAZEPOXI<br />
DE 10 mg Tablet Oral<br />
1221 LIDOCAINE LIDOCAINE 2%w/w Gel Topical<br />
1222 LIDOCAINE LIDOCAINE 2% w/v<br />
1223 LIFETONE<br />
1224 LIFETONE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
AMINO<br />
ACIDS+FOLIC<br />
ACID+IRON+VITB.<br />
12 Multiple<br />
1225 LIGNOCAINE LIDOCAINE 2% w/v/ 5 ml<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
1226 LIGNOCAINE LIDOCAINE 2% w/v/ Gel Topical<br />
1227 LIPITOR<br />
ATORVASTATIN<br />
CALCIUM 10 mg Tablet Oral<br />
CADILA<br />
(HEALTHCAR<br />
E)<br />
PHARMACEUT<br />
ICALS LTD.<br />
CADILA<br />
(HEALTHCAR<br />
E)<br />
PHARMACEUT<br />
ICALS LTD.<br />
CADILA<br />
(HEALTHCARE)<br />
PHARMACEUTICALS<br />
LTD.<br />
CADILA<br />
(HEALTHCARE)<br />
PHARMACEUTICALS<br />
LTD.<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 8/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 8/1/2011<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 11/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
ABYAK<br />
PHARMA LTD.<br />
ABYAK<br />
PHARMA LTD.<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
ABYAK PHARMA<br />
LTD. 12/1/2012<br />
ABYAK PHARMA<br />
LTD. 12/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 3/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
PFIZER WEST<br />
AFRICA (NIGERIA)<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
142<br />
MARCH 1, 2011
1228 LIPITOR<br />
ATORVASTATIN<br />
CALCIUM 20 mg Tablet Oral<br />
1229 LISCARD-10 LISINOPRIL 10 mg Tablet Oral<br />
1230 LISCARD-5 LISINOPRIL 5 mg Tablet Oral<br />
1231 LISINACE LISINOPRIL 5 mg Tablet Oral<br />
1232 LISINACE LISINOPRIL 10 mg Tablet Oral<br />
1233 LISINOP LISINOPRIL 5 mg Tablet Oral<br />
1234 LISINOP LISINOPRIL 10 mg Tablet Oral<br />
1235 LISINOPRIL LISINOPRIL 5 mg Tablet Oral<br />
1236 LISINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
PFIZER WEST<br />
AFRICA (NIGERIA)<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
FAR EAST<br />
MERCANTILE 4/1/2012<br />
FAR EAST<br />
MERCANTILE 4/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
143<br />
MARCH 1, 2011
1237 LISINOPRIL LISINOPRIL 20 mg Tablet Oral<br />
1238 LISINOPRIL LISINOPRIL 5 mg Tablet Oral<br />
1239 LISINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
1240 LISINOPRIL LISINOPRIL 20 mg Tablet Oral<br />
1241 LISINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
1242 LISINOPRIL LISINOPRIL 20 mg Tablet Oral<br />
1243 LISINOPRIL LISINOPRIL 5 mg Tablet Oral<br />
1244 LISINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
1245 LISINOPRIL LISINOPRIL 5 mg Tablet Oral<br />
1246 LISINOPRIL LISINOPRIL 20 mg Tablet Oral<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
144<br />
MARCH 1, 2011
1247 LISINOPRIL LISINOPRIL 5 mg Tablet Oral<br />
1248 LISINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
1249 LISINOPRIL LISINOPRIL 20 mg Tablet Oral<br />
1250 LISINOVA LISINOPRIL 5 mg Tablet Oral<br />
1251 LISINOVA LISINOPRIL 10 mg Tablet Oral<br />
1252 LISINOVA LISINOPRIL 20 mg Tablet Oral<br />
1253<br />
LISTERINE<br />
(COOLMINT)<br />
1254 LISTERINE (ORIGINAL)<br />
1255<br />
LISTERINE TEETH &<br />
GUM DEFENCE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
MENTHOL+METHY<br />
L<br />
SALICYLATE+OTH<br />
ERS Multiple Oral Solution<br />
MENTHOL+METHY<br />
L<br />
SALICYLATE+OTH<br />
ERS Multiple Oral Solution<br />
FLUORIDE+ALCOH<br />
OL Multiple<br />
Mouthwashes<br />
(oral rinse)<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PARKE DAVIS,<br />
UK<br />
PARKE DAVIS,<br />
UK<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
PHARMANOVA<br />
LTD.<br />
PHARMANOVA<br />
LTD.<br />
PHARMANOVA<br />
LTD.<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
JOHNSON &<br />
JOHNSON SUB-<br />
SAHAR AFRICA<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
UNICHEM<br />
GHANA LTD. 10/1/2012<br />
UNICHEM<br />
GHANA LTD. 10/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 4/1/2012<br />
145<br />
MARCH 1, 2011
1256 LISTRIL LISINOPRIL 10 mg Tablet Oral<br />
1257 LISTRIL LISINOPRIL 5 mg Tablet Oral<br />
1258<br />
LIVERTONE JUNIOR<br />
BLOOD TONIC<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
1259 LIVIAL TIBOLONE 2.5 mg Tablet Oral<br />
1260 LIVOLIN FORTE<br />
PHOSPHATIDYLCH<br />
OLINE+VIT.<br />
B1+VIT B2+VIT.<br />
B3+VIT 6+VIT<br />
B12+VIT Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1261 LIVOSTIN EYE LEVOCABASTINE 0.50 mg/ml Eye drops<br />
1262 LIVOSTIN NASAL LEVOCABASTINE 0.50 mg/ml<br />
Topical<br />
Spray<br />
1263 LNG IUS LEVONORGESTREL 52.00 mg Implant<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED<br />
(INDIA)<br />
JANSSEN-<br />
CILAG<br />
(SWITZERLAN<br />
D)<br />
JANSSEN-<br />
CILAG<br />
(SWITZERLAN<br />
D)<br />
BAYER -<br />
SCHERING<br />
PHARMA OY<br />
(FINLAND)<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 11/1/2012<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 2/1/2013<br />
ERNEST CHEMISTS<br />
LIMITED<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED (INDIA)<br />
JANSSEN-CILAG<br />
(SWITZERLAND)<br />
JANSSEN-CILAG<br />
(SWITZERLAND)<br />
BAYER - SCHERING<br />
PHARMA OY<br />
(FINLAND)<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 11/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 8/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 8/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 3/1/2013<br />
146<br />
MARCH 1, 2011
1264 LOCID-20 OMEPRAZOLE 20 mg<br />
1265 LOCORTEN-VIOFORM<br />
CLIOQUINOL+FLU<br />
METHASONE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1%w/v/0.02%<br />
w/v Ear Drops<br />
1266 LOFNAC DICLOFENAC 100 mg Tablet Oral<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
1267 LOFNAC DICLOFENAC 50 mg Tablet Oral GVS LABS GVS LABS<br />
1268 LOFNAC<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE Menthol Gel Topical GVS LABS GVS LABS<br />
1269 LOFNAC DICLOFENAC 100 mg Suppository<br />
1270 LOFNAC DICLOFENAC 75 mg/3 ml<br />
Injectable<br />
Solution<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
1271 LOFNAC DICLOFENAC 50 mg Suppository GVS LABS GVS LABS<br />
1272 LOFNAC-P<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
1273 LOMAC OMEPRAZOLE 20 mg<br />
50 mg/500<br />
mg Tablet Oral GVS LABS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
BIO ETHICALS<br />
PHARMA LTD<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 10/4/2013<br />
TOBINCO<br />
PHARMACY 9/1/2011<br />
TOBINCO<br />
PHARMACY 11/10/2011<br />
TOBINCO<br />
PHARMACY 11/1/2011<br />
TOBINCO<br />
PHARMACY 11/1/2011<br />
TOBINCO<br />
PHARMACY 9/1/2011<br />
TOBINCO<br />
PHARMACY 7/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 8/1/2012<br />
147<br />
MARCH 1, 2011
1274 LOMODEM LOPERAMIDE 2 mg<br />
1275 LONART<br />
1276 LONART<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
1277 LOPERAMIDE LOPERAMIDE 2 mg<br />
1278 LOPERAMIDE LOPERAMIDE 2 mg<br />
1279 LOPERAMIDE DENK LOPERAMIDE 2 mg<br />
1280<br />
1281<br />
LOPINAVIR&RITONAVI<br />
R<br />
LOPINAVIR+RITONAVI<br />
R<br />
LOPINAVIR+RITON<br />
AVIR<br />
LOPINAVIR+RITON<br />
AVIR<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
20 mg/120<br />
mg Tablet Oral<br />
180 mg/1080<br />
mg/60 mls<br />
200 mg/50<br />
mg<br />
200 mg + 50<br />
mg<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
1282 LORSIPOL LISINOPRIL 5 mg Tablet Oral<br />
ELINDA<br />
PHARMACY UNI-MED INDIA<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
BLISS GVS<br />
PHARMA LTD GVS LABS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
VICTORY<br />
PHARMACY 8/1/2011<br />
TOBINCO<br />
PHARMACY 8/20/2011<br />
TOBINCO<br />
PHARMACY 8/20/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 12/1/2012<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2013<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
148<br />
MARCH 1, 2011
1283 LOSACAR LOSARTAN 50 mg Tablet Oral<br />
1284 LOSAR LOSARTAN 50 mg Tablet Oral<br />
1285 LOSAR H<br />
HYDROCHLOROTH<br />
IAZIDE+LOSARTAN<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
12.5 mg + 50<br />
mg Tablet Oral<br />
1286 LOSARTAS LOSARTAN 50 mg Tablet Oral<br />
1287<br />
1288<br />
1289<br />
1290<br />
LUEX ADULT DRY<br />
COUGH<br />
LUEX BABY CHESTY<br />
COUGH<br />
LUEX CHILDREN<br />
CHESTY COUGH<br />
LUEX CHILDREN'S DRY<br />
COUGH<br />
DEXTROMETHORP<br />
HAN+MENTHOL<br />
10 mg/2.4<br />
mg/5 mls Syrup<br />
AMMONIUM<br />
CHLORIDE+CHLOR<br />
PHENIRAMINE+ME<br />
NTHOL Multiple Syrup<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE+ME<br />
NTHOL+SODIUM<br />
CITRATE Multiple Syrup<br />
CHLORPHENIRAMI<br />
NE+DEXTROMETH<br />
ORPHAN 10 mg/4 mg Syrup<br />
CADILA<br />
(HEALTHCAR<br />
E)<br />
PHARMACEUT<br />
ICALS LTD.<br />
CADILA<br />
(HEALTHCARE)<br />
PHARMACEUTICALS<br />
LTD.<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD-MATODA<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
LI TAKA<br />
PHARMACEUTICALS<br />
LTD.<br />
AGLOWMED<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 8/1/2011<br />
GR PHARMA<br />
LTD. 2/1/2013<br />
GR PHARMA<br />
LTD. 2/1/2013<br />
GR PHARMA<br />
LTD. 10/1/2012<br />
UNICHEM<br />
GHANA LTD. 9/1/2012<br />
UNICHEM<br />
GHANA LTD. 9/1/2012<br />
UNICHEM<br />
GHANA LTD. 9/1/2012<br />
UNICHEM<br />
GHANA LTD. 9/1/2012<br />
149<br />
MARCH 1, 2011
1291<br />
LUEX NIGHT TIME<br />
COUGH<br />
1292 LUMAGLOBE 480<br />
1293 LUMAGLOBE120<br />
1294 LUMAQUEL<br />
1295 LUMARTEM<br />
1296 LUMERAX<br />
1297 LUMET<br />
1298 LUMET DT<br />
1299 LUMETHER-140<br />
1300 LUMINATE<br />
1301 LUSIA-F<br />
DEXTROMETHORP<br />
HAN+MENTHOL+P<br />
SEUDOEPHEDRINE<br />
+TRIPROLIDINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER +<br />
LUMEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ETHINYLESTRADIO<br />
L+IRON+NORGEST<br />
REL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
10 mg+ 1.5<br />
mg + 30 mg +<br />
1.25 mg Syrup<br />
80 mg+ 480<br />
mg Tablet Oral<br />
20 mg+ 120<br />
mg Tablet Oral<br />
20 mg+120<br />
mg<br />
Tablet<br />
Chewable<br />
20 mg/120<br />
mg Tablet Oral<br />
20 mg/120<br />
mg Tablet Oral<br />
20 mg + 120<br />
mg Tablet Oral<br />
20 mg + 120<br />
mg Tablet Oral<br />
20 mg/120<br />
mg Tablet Oral<br />
20 mg + 120<br />
mg Tablet Oral<br />
0.3 mg/0.03<br />
mg/75 mg<br />
Tablet film<br />
coated<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
MERCURY<br />
ANTIBIOTICS PVT.<br />
LTD.<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
SEQUEL<br />
PHARMACHEM PVT<br />
LTD.<br />
UNICHEM<br />
GHANA LTD. 5/10/2013<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/21/2015<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/21/2015<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 7/22/2015<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 11/1/2012<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
XL LABORATORIES<br />
PVT LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
PHARMANOV<br />
A LTD.<br />
FAMY CARE<br />
LIMITED<br />
PHARMANOVA<br />
LTD.<br />
FAMY CARE<br />
LIMITED<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 10/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 11/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 11/1/2012<br />
KINAPHARMA<br />
LTD 8/1/2011<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
GSMF<br />
INTERNATIONAL 6/1/2013<br />
150<br />
MARCH 1, 2011
1302 LUTHER 80/2 ARTEMETHER 40 mg/ml<br />
1303 LYCLEAR DERMAL PERMETHRIN 5%w/w<br />
1304 LYCORTIN-S HYDROCORTISONE 100 mg/vial<br />
1305 LYFAXONE CEFTRIAXONE 1 g<br />
1306 LYKTOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Cream<br />
Topical<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE Multiple Lozenges<br />
1307 LYRICA PREGABALIN 150 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
LYKA LABS.<br />
LIMITED<br />
SURGECARE<br />
& PHARMA<br />
LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
LYKA LABS.<br />
LIMITED<br />
LYKA LABS.<br />
LIMITED<br />
SMOOTH<br />
ESKAY AYUVERDIC<br />
THERAPEUTIC PHARMACY PVT.<br />
S<br />
LTD.<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
PFIZER GmbH<br />
ARNEIMITTELWERK<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTIC<br />
ALS PVT LTD 12/31/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 8/1/2013<br />
SURGECARE &<br />
PHARMA<br />
LIMITED 5/1/2012<br />
ESKAY<br />
THERAPEUTICS 5/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 2/24/2013<br />
151<br />
MARCH 1, 2011
1308 MABTHERA RITUXIMAB<br />
1309 MAGNAVIT<br />
1310 MAGNEVIST<br />
1311 MAGTRISIL<br />
1312 MALADOXINE<br />
1313 MALAR-2<br />
1314 MALAR-2<br />
500 mg/50<br />
ml<br />
MULTIVITAMIN+MI<br />
NERALS Mulitple<br />
GADOPENTETATE<br />
DIMEGLUMINE<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
TRISILICATE<br />
PYRIMETHAMINE+<br />
SULPHADOXINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
0.50<br />
mmol/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
120 mg/250<br />
mg Tablet Oral<br />
500 mg/25<br />
mg Tablet Oral<br />
20 mg/120<br />
mg Tablet Oral<br />
15 mg/90<br />
mg/5 mls<br />
Powder for<br />
Oral<br />
Suspension<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ)<br />
AGGENENTECH<br />
INC (SAN<br />
FRANCISCO)GENE<br />
NTECH INC<br />
(VACAVILLE)<br />
SHALINA<br />
LABORATORIE SOFTSULE PVT<br />
S PVT. LTD. LTD<br />
N.V.<br />
SCHERING<br />
S.A<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
BAYER - SCHERING<br />
PHARMA<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
UNI-MED<br />
INDIA UNI-MED INDIA<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 9/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 11/1/2012<br />
BAYER -<br />
SCHERING<br />
Ghana office. 8/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
VICTORY<br />
PHARMACY 4/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
152<br />
MARCH 1, 2011
1315 MALARID<br />
1316 MALIN COUGH<br />
1317 MALIN JUNIOR COUGH<br />
1318 MALMED ADULT<br />
PYRIMETHAMINE+<br />
SULPHADOXINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg/25<br />
mg Tablet Oral<br />
ANISEED<br />
OIL+CAPSICUM+E<br />
UCALYPTUS+LIQU<br />
ORICE+MENTHOL+<br />
OTHERS Multiple Lozenges<br />
DIPHENHYDRAMIN<br />
E+MENTHOL+SODI<br />
UM CITRATE<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
1319 MARCAINE BUPIVACAINE 0.5% w/v<br />
1320<br />
MARCAINE SPINAL<br />
HEAVY<br />
1321 MASCOUGH<br />
1322 MAXAPAC<br />
BUPIVACAINE+GL<br />
UCOSE<br />
12.5 mg/1.25<br />
mg/25 mg Syrup<br />
100 mg/300<br />
mg Tablet Oral<br />
5 mg/80<br />
mg/ml<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
AMMONIUM<br />
CHLORIDE+CHLOR<br />
PHENIRAMINE+DE<br />
XTROMETHORPHA<br />
N+OTHER Multiple Syrup<br />
ASPIRIN +<br />
CAFFEINE+<br />
PARACETAMOL<br />
150 mg+ 30<br />
mg+250 mg Tablet Oral<br />
PHARMANOV<br />
A LTD.<br />
POKU<br />
PHARMA LTD.<br />
POKU<br />
PHARMA LTD.<br />
MEDINOMICS<br />
HEALTHCARE<br />
PRIVATE LTD<br />
ASTRAZENEC<br />
A<br />
AB(Sweden)<br />
ASTRAZENEC<br />
A<br />
AB(Sweden)<br />
DAAMASS CO.<br />
LIMITED<br />
UNICHEM<br />
INDUSTRIES<br />
LTD<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
PHARMANOVA<br />
LTD. 7/1/2012<br />
M & G<br />
PHARMACEUTICALS<br />
LTD 2/1/2013<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
POKU PHARMA<br />
LTD. 10/1/2012<br />
MADRAS<br />
PHARMACEUTICALS KENDICKS<br />
PHARMACY 9/1/2011<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
MERCURY<br />
HEALTHCARE PVT.<br />
LTD<br />
UNICHEM<br />
INDUSTRIES LTD<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 6/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 6/1/2011<br />
DAAMASS CO.<br />
LIMITED 9/1/2011<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
153<br />
MARCH 1, 2011
1323 MAXIMYCIN AZITHROMYCIN I g<br />
1324 MAXIMYCIN AZITHROMYCIN 200 mg<br />
1325 MAXIRON<br />
1326 MAXIRON PLUS<br />
1327 MAXITROL<br />
1328 MAXITROL<br />
1329 MAXOLON<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Powder for<br />
Oral<br />
Suspension<br />
IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX 60 mg/5 ml Syrup<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
60 mg/1.5<br />
mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DEXAMETHASONE<br />
+NEOMYCIN+POLY<br />
MYXIN B Multiple Eye ointment<br />
DEXAMETHASONE<br />
+NEOMYCIN+POLY<br />
MYXIN B Multiple Eye drops<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 5.0 mg/ml<br />
Injectable<br />
Solution<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
ROCK<br />
CHEMISTS<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 9/1/2011<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD 6/1/2011<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
ALCON<br />
PHARMACEUTICALS<br />
ALCON<br />
PHARMACEUTICALS<br />
OSON'S CHEMIST<br />
LTD 12/1/2012<br />
OSON'S CHEMIST<br />
LTD 5/1/2013<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 7/23/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 7/22/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
154<br />
MARCH 1, 2011
1330 MB-CHLOR EYE<br />
CHLORAMPHENIC<br />
OL 0.5%w/w Eye drops<br />
1331 M-CAM MELOXICAM 7.5 mg Tablet Oral<br />
1332 M-CAM MELOXICAM 15 mg Tablet Oral<br />
1333<br />
1334<br />
1335<br />
MEASLES, MUMPS AND<br />
RUBELLA<br />
VACCINE(ATTENUATE<br />
D)<br />
MEDICATED<br />
MENTHOLATED<br />
DUSTING<br />
MEDI-KEEL A<br />
BLACKCURRANT<br />
THROAT LOZENGES<br />
MEASLES+MUMPS+<br />
RUBELLA<br />
1000<br />
CCID50/5000<br />
CCID50/1000<br />
CCID50/dose<br />
MENTHOL+ZINC<br />
OXIDE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Powder for<br />
topical use<br />
CETYLPYRIDIUM+L<br />
IGNOCAINE 12.0 + 1.5mg Lozenges<br />
1336 MEDIPRIST MIFEPRISTONE 200 mg Tablet Oral<br />
1337 MEDISOFT<br />
TRICHLOROCARBA<br />
NILIDE Solid Soap<br />
1338 MEDITRAX CEFTRIAXONE 1 gm<br />
Injectable<br />
Powder<br />
MULTINATION<br />
AL BUSINESS<br />
LINK<br />
S. J. & G FAZUL<br />
ELLAHIE (PVT)<br />
LTD.<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
SERUM<br />
INSTITUTE OF SERUM INSTITUTE<br />
INDIA OF INDIA<br />
PZ CUSSONS<br />
GH. LTD<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)PZ<br />
CUSSONS GH. LTD<br />
UNIQUE<br />
PHARMACEUTICAL<br />
LABORATORIES<br />
SALOM<br />
PHARMACY<br />
LIMITED 12/1/2012<br />
GR PHARMA<br />
LTD. 11/1/2013<br />
GR PHARMA<br />
LTD. 11/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 7/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
CIPLA<br />
LIMITED CIPLA LIMITED GOKALS LTD 3/1/2012<br />
PEACE INDO<br />
GHANA<br />
MEDITAB<br />
SPECIALITIES.<br />
PVT. LTD<br />
PEACE INDO<br />
GHANA<br />
MEDITAB<br />
SPECIALITIES. PVT.<br />
LTD<br />
PEACE INDO<br />
GHANA 9/24/2012<br />
SPINTEX<br />
CHEMIST LTD 11/1/2013<br />
155<br />
MARCH 1, 2011
1339 MEDITROL CALCITRIOL 0.25 mcg<br />
1340 MEFART<br />
1341 MEFART<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
ARTEMETHER+LU<br />
MEFANTRINE<br />
15 mg + 90<br />
mg/per 5ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Softgel)<br />
Powder for<br />
Oral<br />
Suspension<br />
1342 MEFENAMIC ACID MEFENAMIC ACID 500 mg Tablet Oral<br />
1343 MELONAX MELOXICAM 15 mg Tablet Oral<br />
1344 MELPROX PIROXICAM 20 mg<br />
1345 MENCEVAX ACWY<br />
MENINGITIS<br />
VACCINE Multiple<br />
1346 MENTEL MEBENDAZOLE 500 mg<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Tablet<br />
Chewable<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 9/1/2012<br />
PHARMANOV<br />
A LTD. 6/1/2013<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
SMITHKLINE<br />
BEECHAM<br />
BIOLOGICALS<br />
(BELGIUM)<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
SMITHKLINE<br />
BEECHAM<br />
BIOLOGICALS(BELG<br />
IUM)<br />
DANDONG<br />
PHARMACEUTICAL<br />
FACTORY<br />
OSON'S CHEMIST<br />
LTD 7/2/2015<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 7/1/2011<br />
BONS<br />
PHARMACY LTD 5/1/2013<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 7/1/2012<br />
156<br />
MARCH 1, 2011
1347 MENTHOCEP<br />
1348 MENTHODEX<br />
1349 MENTHODEX<br />
1350 MENTHOLATUM BALM<br />
AMMONIUM<br />
CHLORIDE+MENTH<br />
OL+SODIUM<br />
CITRATE+OTHERS Multiple Syrup<br />
EUCALYPTUS+MEN<br />
THOL Lozenges<br />
AMMONIUM<br />
CHLORIDE+MENTH<br />
OL+SODIUM<br />
CITRATE+OTHERS Multiple Syrup<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
1351 MEPRAZ OMEPRAZOLE 20 mg<br />
1352<br />
MERCUROCROME<br />
PAINT MERCUROCHROME 0.5%w/v<br />
1353 MEROLAG-S ESOMEPRAZOLE 40 mg<br />
1354 MEROLAG-S<br />
ESOMEPRAZOLE<br />
MAGNESIUM<br />
TRIHYDRATE 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Capsule<br />
Delayed<br />
Release<br />
Topical<br />
solution<br />
Capsule with<br />
Enteric<br />
coated<br />
granules<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
MENTHOLATU<br />
M COMPANY<br />
LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
GEO MEDICORE<br />
LIMITED<br />
BELLS & SONS &<br />
CO LTD<br />
BELLS & SONS &<br />
CO LTD<br />
MENTHOLATUM<br />
COMPANY LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
KAMA INDUSTRIES<br />
LTD.<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
GEO MEDICORE<br />
LIMITED 12/31/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
GB PHARMA<br />
(GH) LTD 12/31/2011<br />
OSON'S CHEMIST<br />
LTD 12/31/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 11/1/2011<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 11/1/2011<br />
157<br />
MARCH 1, 2011
1355 MERONEM MEROPENEM 1 g<br />
1356 MERONEM MEROPENEM 500 mg<br />
1357 METAKELFIN<br />
PYRIMETHAMINE+<br />
SULPHAMETHOXY<br />
PYRAZINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
500 mg/25<br />
mg Tablet Oral<br />
1358 METASOL MONOSULPIRAM 5.00 %w/w Solid Soap<br />
1359 METFOMIN METFORMIN 500 mg Tablet Oral<br />
1360 METFORMIN METFORMIN 500 mg Tablet Oral<br />
1361 METFORMIN METFORMIN 500 mg Tablet Oral<br />
1362 METFORMIN METFORMIN 500 mg Tablet Oral<br />
ZENECA<br />
PHARMACEUT<br />
ICALS<br />
ZENECA<br />
PHARMACEUT<br />
ICALS<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
ASTRAZENECA UK<br />
LTDSUMITOMO<br />
PHARMACEUTICALS<br />
CO. LTD<br />
ASTRAZENECA UK<br />
LTDZENECA<br />
PHARMACEUTICALS<br />
SUMITOMO<br />
PHARMACEUTICALS<br />
CO. LTD<br />
PFIZER ITALIA S.r.<br />
L.<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 4/1/2012<br />
ELLIOT<br />
IRVING<br />
LIMITED SIGMA SOAP LTD. PNT PHARMACY 11/1/2011<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 4/1/2012<br />
158<br />
MARCH 1, 2011
1363 METFORMIN DENK 500 METFORMIN 500 mg Tablet Oral<br />
1364 METFORMIN DENK 850 METFORMIN 850 mg Tablet Oral<br />
1365 METFORMIN-DOR METFORMIN 500 mg Tablet Oral<br />
1366 METHYLDOPA METHYLDOPA 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
1367 METHYLDOPA METHYLDOPA 250 mg Tablet Oral<br />
1368 METHYLDOPA METHYLDOPA 250 mg<br />
Tablet film<br />
coated<br />
1369 METMIN METFORMIN 500 mg Tablet Oral<br />
1370 METOCLOPRAMIDE<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 10 mg Tablet Oral<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
SHIJIAZHUANG<br />
PHARMA. GROUP<br />
ZHONGNUO<br />
PHARMACEUTICAL<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
GRACURE<br />
PHARMACEUT<br />
ICALS LTD<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
GRACURE<br />
PHARMACEUTICALS<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 6/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
SALOM<br />
PHARMACY<br />
LIMITED 9/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
159<br />
MARCH 1, 2011
1371 METOCLOPRAMIDE<br />
1372 METOCLOPRAMIDE<br />
1373 METOCLOPRAMIDE<br />
1374 METOCLOPRAMIDE<br />
1375 METOCLOPRAMIDE<br />
1376<br />
1377<br />
1378<br />
1379<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 10 mg Tablet Oral<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 10 mg Tablet Oral<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 5.0 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 10 mg Tablet Oral<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 10 mg Tablet Oral<br />
METOCLOPRAMIDE<br />
HYDROCHLORIDE 5.0 mg/ml<br />
Injectable<br />
Solution<br />
METOPROLOL<br />
HYDROCHLORIDE METOPROLOL 50 mg Tablet Oral<br />
METOPROLOL<br />
HYDROCHLORIDE METOPROLOL 100 mg Tablet Oral<br />
METOPROLOL<br />
HYDROCHLORIDE METOPROLOL 50 mg Tablet Oral<br />
BEDITA<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROCK<br />
CHEMISTS<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
WUHAN GRAND<br />
PHARM GROUP CO.<br />
LTD. ROCK CHEMISTS 2/1/2012<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
160<br />
MARCH 1, 2011
1380 METRO METRONIDAZOLE 125 mg/5 mls<br />
1381 METROFORTE-F<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE 100+400 mg Tablet Oral<br />
1382 METROKIN METRONIDAZOLE 200 mg Tablet Oral<br />
1383 METROLEX-F<br />
1384 METROLEX-F<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
150.00<br />
mg/30 mg<br />
1385 METRONIDAZOLE METRONIDAZOLE 0.5 %W/V<br />
1386 METRONIDAZOLE METRONIDAZOLE 0.5%w/v<br />
Oral<br />
Suspension<br />
100 mg + 400<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
1387 METRONIDAZOLE METRONIDAZOLE 200 mg Tablet Oral<br />
1388<br />
METRONIDAZOLE<br />
BENZOATE METRONIDAZOLE 200 mg Tablet Oral<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
UNICHEM<br />
GHANA LTD.<br />
FRESENIUS<br />
KABI INDIA<br />
PVT. LIMITED<br />
GUANDONG<br />
MEDICINES &<br />
HEALTH<br />
PRODUCTS<br />
I/E CORP<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
UNICHEM GHANA<br />
LTD.<br />
FRESENIUS KABI<br />
INDIA PVT.<br />
LIMITED<br />
SISHUI XIERKANG<br />
PHARMACEUTICAL<br />
COMPANY<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
SALOM<br />
PHARMACY<br />
LIMITED 10/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 8/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
UNICHEM<br />
GHANA LTD. 8/1/2011<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
161<br />
MARCH 1, 2011
1389<br />
METRONIDAZOLE<br />
BENZOATE METRONIDAZOLE 125 mg/5 ml<br />
1390 METRONIDAZOLE I.V. METRONIDAZOLE<br />
1391<br />
METROPLUS JUNIOR<br />
SUSPENSION<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
500 mg/100<br />
ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
150 mg/30<br />
mg/5 mls Syrup<br />
1392 METROSULE METRONIDAZOLE 200 mg Tablet Oral<br />
1393 METROZONE<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
150 mg/30<br />
mg/5ml<br />
Oral<br />
Suspension<br />
1394 METZ METFORMIN 500 mg Tablet Oral<br />
1395 MEZOL-MF<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
150 mg/30<br />
mg/5 mls<br />
1396 MEZOLYN MEROPENEM 500 mg<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
ENICAR<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD.<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
GR PHARMA<br />
LTD. 7/1/2012<br />
ENICAR<br />
PHARMACEUTICALS<br />
PVT LTD. PLUS PHARMACY 3/6/2050<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
UNI-MED<br />
INDIA UNI-MED INDIA<br />
EROS<br />
PHARMA LTD.<br />
R H R<br />
MEDICARE P.<br />
LTD<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
SCIENTIFIC<br />
OFFICE (NIG)<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
R H R MEDICARE P.<br />
LTD<br />
SANDOZ PRIVATE<br />
LTD<br />
NEO PHARMA<br />
CENTRE LIMITED 6/1/2013<br />
VICTORY<br />
PHARMACY 11/1/2011<br />
FAR EAST<br />
MERCANTILE 9/1/2011<br />
HOVID<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 11/1/2013<br />
162<br />
MARCH 1, 2011
1397 MEZOLYN MEROPENEM 1 g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
1398 MICLOFEN DICLOFENAC 100 mg Tablet Oral<br />
1399 MICOTRIN MICONAZOLE 2 % W/W<br />
1400 MICROGYNON<br />
Cream<br />
Topical<br />
ETHINYLESTRADIO<br />
L+LEVONORGESTR 0.15 mg/0.03<br />
EL<br />
mg Tablet Oral<br />
1401 MIDIPINE NIFEDIPINE 20 mg<br />
1402 MILPAR<br />
PARAFFIN+MAGNE<br />
SIUM HYDROXIDE<br />
Tablet<br />
Extended<br />
Realese<br />
25%w/v/6%w<br />
/v Oral Solution<br />
1403 MIMET ERGOMETRINE 0.5 mg Tablet Oral<br />
1404 MIMORAL<br />
1405 MIMOVITE<br />
CHYMOTRYPSIN+T<br />
RYPSIN 50,000USP Tablet Oral<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Caplets oral<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
SCIENTIFIC<br />
OFFICE (NIG)<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
SANDOZ PRIVATE<br />
LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
BAYER - SCHERING<br />
PHARMA<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
PALB<br />
PHARMACEUTIC<br />
ALS 11/1/2013<br />
ESKAY<br />
THERAPEUTICS 5/1/2013<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
OSON'S CHEMIST<br />
LTD 4/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
163<br />
MARCH 1, 2011
1406 MINAVITA MULTIVITAMIN Multiple Syrup<br />
1407 MIRCERA<br />
1408 MIRCERA<br />
1409 MIRCERA<br />
METHOXY<br />
POLYETHYLENE<br />
GLYCOL-EPOETIN<br />
BETA<br />
METHOXY<br />
POLYETHYLENE<br />
GLYCOL-EPOETIN<br />
BETA<br />
METHOXY<br />
POLYETHYLENE<br />
GLYCOL-EPOETIN<br />
BETA<br />
50 mcg/0.3<br />
mls<br />
75 mcg/0.3<br />
mls<br />
200 mcg/0.3<br />
mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Suspension<br />
Injectable<br />
Suspension<br />
Injectable<br />
Suspension<br />
1410 MIRENA LEVONORGESTREL 52 mg Implant<br />
1411 MISAVITE 100 VITAMIN E 100 mg/iu<br />
1412 MISAVITE 200 VITAMIN E 200 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
F. HOFFMANN-<br />
LA ROCHE<br />
LTD.(Switzerl<br />
and)<br />
F. HOFFMANN-<br />
LA ROCHE<br />
LTD.(Switzerl<br />
and)<br />
F. HOFFMANN-<br />
LA ROCHE<br />
LTD.(Switzerl<br />
and)<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
AFRICA GMBH<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
BAYER - SCHERING<br />
PHARMA OY<br />
(FINLAND)<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 3/1/2012<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 8/18/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 8/18/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 8/18/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
OSON'S CHEMIST<br />
LTD 10/1/2013<br />
OSON'S CHEMIST<br />
LTD 10/1/2013<br />
164<br />
MARCH 1, 2011
1413 MISOTAC MISOPROSTOL 200 mcg Tablet Oral<br />
1414 MISSION'S B COMPLEX<br />
1415<br />
VITAMIN B<br />
COMPLEX Multiple<br />
MIXTARD 30 HM<br />
PENFILL HUMAN INSULIN 100 iu/10 ml<br />
1416 MOLFEN<br />
1417<br />
MORGANS ANTISEPTIC<br />
MEDICATED<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Suspension<br />
325 mg/400<br />
mg Caplets oral<br />
SODIUM<br />
TALLOWATE Solid Soap<br />
1418 MORPHINE MORPHINE 10 mg<br />
1419 MORPHINE MORPHINE 10 mg/ml<br />
1420 MORPHINE-FRESENIUS MORPHINE 10 mg/ ml<br />
1421 MORTIN FORTE<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
Tablet<br />
Extended<br />
Realese<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
800 mg+160<br />
mg Tablet Oral<br />
SIGMA<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRIES<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
ALDAPH SPA<br />
(NOVO<br />
NORDISK<br />
ALGERIA)<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
MORGAN'S<br />
POMADE CO.<br />
LTD<br />
SUBVICK<br />
CHEMICALS,<br />
UK<br />
SIGMA<br />
PHARMACEUTICAL<br />
INDUSTRIES<br />
MISSION VIVACARE<br />
LIMITED<br />
CYNDEX<br />
PHARMACY 10/1/2011<br />
OSON'S CHEMIST<br />
LTD 2/1/2012<br />
ALDAPH SPA (NOVO<br />
NORDISK<br />
ALGERIA) DANAFCO LTD 4/1/2011<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
MORGAN'S POMADE<br />
CO. LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 2/1/2013<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
MICRO<br />
EXPORTS(LAB<br />
ORATORIES)<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES)<br />
HILLS<br />
PHARMACY 9/1/2012<br />
J.M. ADDO &<br />
SONS LTD 7/1/2013<br />
165<br />
MARCH 1, 2011
1422 MOSEGOR PIZOTIFEN<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
0.005 g/100<br />
mls Syrup<br />
1423 MOSEGOR PIZOTIFEN 0.5 mg Tablet Oral<br />
1424 MOTILIUM DOMPERIDONE 10 mg Tablet Oral<br />
1425 MOXKING DRY SYRUP AMOXICILLIN 125 mg/5 ml<br />
1426<br />
MSM MULTI<br />
SUPERMAN<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
Oral<br />
Suspension<br />
1427 MUCICLAR ADULTS CARBOCISTEINE 5 %w/v Syrup<br />
1428<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
MUCICLAR FOR<br />
CHILDREN + INFANTS CARBOCISTEINE 2 %w/v Syrup<br />
1429 MUFEN IBUPROFEN 400 mg<br />
NOVARTIS<br />
PHARMA AG FAMAR FRANCE<br />
NOVARTIS<br />
PHARMA AG FAMAR FRANCE<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 11/1/2011<br />
CASSEL<br />
RESEARCH CASSEL RESEARCH<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD PVT. LTD 8/1/2011<br />
UNI-MED<br />
INDIA UNI-MED INDIA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
JOHNSON &<br />
JOHNSON<br />
SUB-SAHAR<br />
AFRICA<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
PARKE-DAVIS<br />
(SENEGAL)JOHNS<br />
ON & JOHNSON<br />
SUB-SAHAR AFRICA<br />
PARKE-DAVIS<br />
(SENEGAL)JOHNS<br />
ON & JOHNSON<br />
SUB-SAHAR AFRICA<br />
MISSION VIVACARE<br />
LIMITED<br />
UNICHEM<br />
GHANA LTD. 2/1/2012<br />
UNICHEM<br />
GHANA LTD. 9/1/2011<br />
UNICHEM<br />
GHANA LTD. 9/1/2011<br />
BONS<br />
PHARMACY LTD 7/1/2013<br />
166<br />
MARCH 1, 2011
1430 MULTIFIT<br />
1431 MULTINOVA<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1432 MULTIVITAMIN MULTIVITAMIN Multiple Tablet Oral<br />
1433 MULTIVITAMIN MULTIVITAMIN Multiple Tablet Oral<br />
1434 MUMFER<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
100 mg/500<br />
mcg<br />
Tablet<br />
Chewable<br />
1435 MUMFER IRON 50 mg/5ml Syrup<br />
1436 MYCLAV 625<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
1437 MYCOLEX MICONAZOLE 2%w/w<br />
1438 MYCOLEX-3<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+NEOMYCIN Multiple<br />
500 mg + 125<br />
mg Tablet Oral<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
FAR EAST<br />
MERCANTILE<br />
PHARMANOV<br />
A LTD.<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
FAR EAST<br />
MERCANTILE 6/1/2012<br />
PHARMANOVA<br />
LTD. 11/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2012<br />
SUPRA PHARMA<br />
LIMITED 6/1/2011<br />
SUPRA PHARMA<br />
LIMITED 6/1/2011<br />
GR PHARMA<br />
LTD. 10/1/2013<br />
UNICHEM<br />
GHANA LTD. 2/1/2013<br />
UNICHEM<br />
GHANA LTD. 8/1/2013<br />
167<br />
MARCH 1, 2011
1439 MYCOPIROX<br />
CICLOPIROX<br />
OLAMINE 1%w/w<br />
1440 MYCOPIROX NAIL CICLOPIROX 8%<br />
1441 MYOGESIC<br />
1442 MYPRODOL<br />
1443 MYPRODOL<br />
CHLORZOXAZONE<br />
+ PARACETAMOL<br />
CODEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
CODEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Vaginal<br />
Topical<br />
solution<br />
250 mg/500<br />
mg Tablet Oral<br />
10 mg+200<br />
mg+250 mg<br />
10 mg+200<br />
mg+250 mg<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1444 MYSOLINE PRIMIDONE 250 mg Tablet Oral<br />
1445 NAKLOFEN DICLOFENAC 50 mg<br />
1446 NAKLOFEN DICLOFENAC 100 mg<br />
Tablet<br />
Enteric<br />
Coated<br />
Tablet<br />
Enteric<br />
Coated<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
ADCOCK<br />
INGRAM<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
PHARMA-<br />
Q(Pty)Ltd.<br />
ADCOCK INGRAM<br />
LIMITED<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 9/1/2012<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 4/1/2013<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
ATLAS<br />
PHARMACY<br />
LIMITED 2/1/2012<br />
ATLAS<br />
PHARMACY<br />
LIMITED 2/1/2012<br />
168<br />
MARCH 1, 2011
1447 NAKLOFEN Duo DICLOFENAC 75 mg<br />
1448 NALOXONE NALOXONE 0.4 mg/ml<br />
1449 NAPHCON- A 2<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule<br />
Extended<br />
Release<br />
Injectable<br />
Solution<br />
NAPHAZOLINE+PH<br />
ENIRAMINE 3.25 mg/ml Eye drops<br />
1450 NAPROSYN EC NAPROXEN 500 mg<br />
1451 NAPROXEN NAPROXEN 500 mg<br />
1452 NAPROXEN NAPROXEN 500 mg<br />
Tablet<br />
Enteric<br />
Coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
1453 NASONEX A.N.S MOMETASONE 0.5% w/v Nasal Spray<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
UNICOM<br />
CHEMIST<br />
LIMITED HIPLUS,S.A<br />
ALCON-<br />
COUVREUR<br />
F. HOFFMANN-<br />
LA ROCHE<br />
LTD.(Switzerl<br />
and)<br />
BEDITA<br />
PHARMACY<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
ALCON-<br />
COUVREUR<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ATLAS<br />
PHARMACY<br />
LIMITED 2/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 12/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
SCHERING-PLOUGH<br />
LABO<br />
N.V.(BELGIUM)SC<br />
HERING-PLOUGH<br />
S.p. A. (ITALY) GOKALS LTD 12/31/2011<br />
169<br />
MARCH 1, 2011
1454 NATRILIX SR INDAPAMIDE 1.50 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Extended<br />
Realese<br />
1455 NELFINEK NELFINAVIR 250 mg Caplets oral<br />
1456 NEOBET-E<br />
1457<br />
SERVIER<br />
EGYPT<br />
INDUSTRY<br />
LTD<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
BETAMETHASONE<br />
AGLOWMED<br />
+NEOMYCIN 5 mg/1 mg/g Eye ointment LIMITED<br />
NEOCIP EYE/EAR<br />
DROPS CIPROFLOXACIN 0.3% w/v<br />
Ear/Eye<br />
Drops<br />
1458 NEOCIP-500 CIPROFLOXACIN 500 mg Tablet Oral<br />
1459 NEO-MEDROL ACNE<br />
METHYLPREDNISO<br />
LONE+NEOMYCIN<br />
2.5 mg/1.75<br />
mg/ml<br />
Topical<br />
Lotion<br />
1460 NEO-MERCAZOLE CARBIMAZOLE 5 mg Tablet Oral<br />
1461 NEO-MERCAZOLE CARBIMAZOLE 5 mg Tablet Oral<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
SERVIER EGYPT<br />
INDUSTRY LTD<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
THE ACME<br />
LABORATORIES<br />
LTD<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OSAKA<br />
PHARMA PVT. OSAKA PHARMA<br />
LTD. PVT. LTD.<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
ROCK<br />
CHEMISTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
OYSTER<br />
HEALTHCARE<br />
LTD. 4/1/2013<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 5/1/2012<br />
PANACEA<br />
PHARMACEUTIC<br />
ALS LTD 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 6/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 2/1/2012<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
170<br />
MARCH 1, 2011
1462 NEO-MERCAZOLE CARBIMAZOLE 5 mg Tablet Oral<br />
1463 NEOMOXINE-P<br />
1464 NEOPEPTINE DROPS<br />
1465 NEOPEPTINE LIQUID<br />
NEOMYCIN+POLY<br />
MYCIN<br />
B+PRAMOXINE<br />
ALPHA<br />
AMYLASE+PAPAIN<br />
+OTHERS<br />
ALPHA<br />
AMYLASE+PAPAIN<br />
+OTHERS<br />
1466 NEOSTIGMINE NEOSTIGMINE 2.5mg/ml<br />
1467 NEOSTIGMINE NEOSTIGMINE 0.5 mg/ml<br />
1468<br />
NEOSTIGMINE<br />
METHYLSULPHATE-<br />
FRESENIUS<br />
1469 NEO-VATE WHITE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
0.5%w/w/0.1<br />
3%w/w/1%w/ Cream<br />
w<br />
Topical<br />
50 mg/100<br />
mg/5 mls Oral Drops<br />
NEOSTIGMINE<br />
METHYLSULPHATE 2.5 mg/1 ml<br />
CLOBETASOL<br />
PROPIONATE 0.05 %w/w<br />
1470 NEPINE SR NIFEDIPINE 20 mg<br />
50 mg/100<br />
mg/5 mls Oral Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Cream<br />
Topical<br />
Tablet<br />
Extended<br />
Realese<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
RAPTAKOS<br />
BRETT & CO.<br />
LIMITED<br />
RAPTAKOS<br />
BRETT & CO.<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
G. D. COOPER &<br />
CO. LIMITED<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
RAPTAKOS BRETT<br />
& CO. LIMITED<br />
RAPTAKOS BRETT<br />
& CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 7/11/2012<br />
FAR EAST<br />
MERCANTILE 3/1/2012<br />
FAR EAST<br />
MERCANTILE 3/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA 2/1/2012<br />
FRESENIUS<br />
KABI (PTY)<br />
LTD (SOUTH<br />
AFRICA)<br />
LACHIFARMA<br />
S.R.L<br />
SMITHKLINE<br />
BEECHAM<br />
INT.<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
LACHIFARMA<br />
S.R.L<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
HILLS<br />
PHARMACY 9/1/2013<br />
K. S. H. CO.<br />
LIMITED 12/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2011<br />
171<br />
MARCH 1, 2011
1471 NERVE AND BONE<br />
1472 NEURVI-DOR<br />
1473 NEUTROSEC<br />
1474 NEUTROSEC<br />
1475 NEUTROTONE WHITE<br />
AMMONIUM<br />
BICARBONATE+AM<br />
MONIUM<br />
CHLORIDE+TURPE<br />
NTINE 12.5%w/v<br />
VITAMIN<br />
B1+VITAMIN B6<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
solution<br />
100 mg + 200<br />
mg Tablet Oral<br />
AMINO<br />
ACID+MULTIVITAM<br />
INS Multiple Syrup<br />
AMINO<br />
ACID+MULTIVITAM<br />
INS Multiple Tablet Oral<br />
CLOBETASOL<br />
PROPIONATE 0.05 %w/w<br />
Cream<br />
Topical<br />
1476 NEVEK NEVIRAPINE 200 mg Caplets oral<br />
1477 NEVIR NEVIRAPINE 200 mg Tablet Oral<br />
1478 NEVIRAPINE NEVIRAPINE 200 mg Tablet Oral<br />
1479 NEVIRAPINE NEVIRAPINE 200 mg Tablet Oral<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
HEBEI<br />
KANGNING<br />
CO . LTD<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
TABLETS<br />
(INDIA)<br />
LIMITED<br />
LACHIFARMA<br />
S.R.L<br />
DANADAMS<br />
PHARMACEUT<br />
ICAL<br />
INDUSTRY<br />
LIMITED<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
BELLS & SONS &<br />
CO LTD<br />
SHIJIAZHUANG<br />
PHARMACEUTICAL<br />
GP CO. LTD<br />
TABLETS (INDIA)<br />
LIMITED<br />
TABLETS (INDIA)<br />
LIMITED<br />
LACHIFARMA<br />
S.R.L<br />
DANADAMS<br />
PHARMACEUTICAL<br />
INDUSTRY LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 10/1/2012<br />
DAAMASS CO.<br />
LIMITED 7/1/2013<br />
DAAMASS CO.<br />
LIMITED 9/1/2013<br />
K. S. H. CO.<br />
LIMITED 7/1/2012<br />
DANADAMS<br />
PHARMACEUTIC<br />
AL INDUSTRY<br />
LIMITED 5/1/2012<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 4/1/2011<br />
STRIDES ARCOLAB<br />
LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2013<br />
172<br />
MARCH 1, 2011
1480 NEW BRAVO<br />
1481 NEWAID BLOOD STOP<br />
1482<br />
NEWFLEX WARMING<br />
RELAXING<br />
CAFFEINE+EPHED<br />
RINE<br />
HCL+PARACETAM<br />
OL<br />
BUTANE+CELLULO<br />
SE<br />
GUM+DISILOXAN Multiple<br />
GAUTHERIA<br />
PROCUMBENS+ME<br />
NTHOL+PINUS<br />
PUMILO OIL<br />
1483 NEWMED EAR Multiple<br />
1484 NEWTONE<br />
1485 NEXCOFER<br />
1486 NEXCOFER<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg+10<br />
mg+ 500 mg Tablet Oral<br />
Topical<br />
Spray<br />
0.175/0.75/0<br />
.675 Gel Topical<br />
Topical<br />
Spray<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
1487 NEXIUM ESOMEPRAZOLE 40 mg Tablet Oral<br />
1488 NEXIUM ESOMEPRAZOLE 20 mg Tablet Oral<br />
GOLDEN<br />
TOWER LTD<br />
GOLDEN TOWER<br />
LTD<br />
NEWPHARMA<br />
AG MEDENA AG<br />
NEWPHARMA<br />
AG MEDENA AG<br />
GOLDEN TOWER<br />
LTD 9/1/2013<br />
SWISS PHARMA<br />
NIGERIA LTD 5/1/2012<br />
SWISSGHA<br />
PHARMA LTD 5/1/2012<br />
NEWPHARMA<br />
AG MEDENA AG 5/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 2/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 2/1/2013<br />
173<br />
MARCH 1, 2011
1489 NEXIUM ESOMEPRAZOLE 10 mg<br />
1490 NEXIUM ESOMEPRAZOLE 40 mg/ml<br />
1491 NIFECARD XL NIFEDIPINE 30 mg<br />
1492<br />
1493<br />
NIFEDI-DENK 10<br />
RETARD NIFEDIPINE 10 mg<br />
NIFEDI-DENK 20<br />
RETARD NIFEDIPINE 20 mg<br />
1494 NIFEDIPINE NIFEDIPINE 10 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Granules For<br />
Oral Use<br />
Injectable<br />
Solution<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Sublingual<br />
1495 NIFEDIPINE NIFEDIPINE 10 mg Tablet Oral<br />
1496 NIFEDIPINE NIFEDIPINE 20 mg Tablet Oral<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED CAMPDALE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED CAMPDALE<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 4/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 4/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 2/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
174<br />
MARCH 1, 2011
1497 NIFEDIPINE NIFEDIPINE 10 mg Tablet Oral<br />
1498 NIFE-DOR NIFEDIPINE 20 mg<br />
1499 NIFE-DOR NIFEDIPINE 10 mg<br />
1500 NIFIN 20 R NIFEDIPINE 20 mg<br />
1501 NIMOTOP NIMODIPINE 30 mg<br />
1502 NIMOTOP NIMODIPINE 0.2%<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet<br />
Delayed<br />
Release<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
1503 NIZORAL KETOCONAZOLE 200 mg Tablet Oral<br />
1504 NIZORAL KETOCONAZOLE 2 % w/w<br />
Cream<br />
Topical<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
VICDORIS<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
BAYER SCHERING<br />
PHARMA AG<br />
BAYER SCHERING<br />
PHARMA AG<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
BAYER - SCHERING<br />
PHARMA<br />
BAYER - SCHERING<br />
PHARMA<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 5/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 5/1/2013<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 10/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 10/1/2013<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 11/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
175<br />
MARCH 1, 2011
1505 NIZORAL SHAMPOO KETOCONAZOLE 2 % w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Topical<br />
Lotion<br />
1506 NOLVADEX-D TAMOXIFEN 20 mg Tablet Oral<br />
1507 NORADRENALINE NORADRENALINE 2 mg/2ml<br />
1508 NORIGYNON<br />
ESTRADIOL+NORE<br />
THISTERONE 5, 50 mg<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
1509 NORLEVO LEVONORGESTREL 750 mcg Tablet Oral<br />
1510 NORMAL SALINE<br />
1511 NORMAL SALINE<br />
1512 NORMORETIC<br />
SODIUM<br />
CHLORIDE 0.9%w/v Eye drops<br />
SODIUM<br />
CHLORIDE 0.9%w/v Nasal Drops<br />
AMILORIDE+HYDR<br />
OCHLORTHIAZIDE Tablet Oral<br />
1513 NOROXIN NORFLOXACIN 400 mg Tablet Oral<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
ASTRAZENEC<br />
A UK LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
LABORATOIRE<br />
S HRA<br />
PHARMA<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
NEIMETH<br />
INT'L PHARM.<br />
PLC<br />
MERCK SHARP<br />
& DOHME<br />
INTERPHARM<br />
A<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
ASTRAZENECA UK<br />
LTD<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
BAYER - SCHERING<br />
PHARMA<br />
CIPLA<br />
LIMITEDCARDINAL<br />
HEALTH FRANCE<br />
429 S.A.S<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
NEIMETH INT'L<br />
PHARM. PLC<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 11/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 11/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 3/17/2014<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 10/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 6/1/2012<br />
MERCK SHARP &<br />
DOHME<br />
INTERPHARMA REISS & CO. 8/1/2012<br />
176<br />
MARCH 1, 2011
1514 NORTH SEA COD LIVER OIL 300 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1515 NORVIR RITONAVIR 80 mg/ml Oral Solution<br />
1516 NO-SPA DROTAVERINE 40 mg Tablet Oral<br />
1517 NO-SPA DROTAVERINE<br />
40<br />
mg/ampoule<br />
Injectable<br />
Solution<br />
1518 NO-SPA FORTE DROTAVERINE 80 mg Tablet Oral<br />
1519 NO-SPALGIN<br />
CODEINE+DROTAV<br />
ERINE+PARACETA<br />
MOL<br />
500 mg/8<br />
mg/40 mg Tablet Oral<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
ABBOTT<br />
LABORATORIE<br />
S S.A. PTY ABBOTT<br />
LTD(South LABORATORIES<br />
Africa) LTD(UK)<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP<br />
(FRANCE)<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP<br />
(FRANCE)<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP<br />
(FRANCE)<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP<br />
(FRANCE)<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 9/27/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
CHINOIN<br />
PHARMACEUTICAL<br />
WORKS CO.<br />
LTDSANOFI-<br />
SYNTHELABO<br />
GROUP (FRANCE) REISS & CO. 9/1/2013<br />
CHINOIN<br />
PHARMACEUTICAL<br />
WORKS CO.<br />
LTDSANOFI-<br />
SYNTHELABO<br />
GROUP (FRANCE) REISS & CO. 8/1/2013<br />
CHINOIN<br />
PHARMACEUTICAL<br />
WORKS CO.<br />
LTDSANOFI-<br />
SYNTHELABO<br />
GROUP (FRANCE) REISS & CO. 9/1/2013<br />
CHINOIN<br />
PHARMACEUTICAL<br />
WORKS CO. LTD REISS & CO. 10/1/2011<br />
177<br />
MARCH 1, 2011
1520 NOSTAMINE<br />
CHLORPHENIRAMI<br />
NE+NAPHAZOLINE<br />
1521 NOVACIP-500 CIPROFLOXACIN 500 mg<br />
1522 NOVACOD COD LIVER OIL 300 mg<br />
1523 NOVACOLD<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
0.5 mg/0.5<br />
mg/ml Eye drops<br />
Tablet film<br />
coated<br />
Capsule oral<br />
(Softgel)<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL+PHE 500 mg/30<br />
NYLPROPANOLAMI mg/2 mg/25<br />
NE<br />
mg Tablet Oral<br />
1524 NOVAFEN IBUPROFEN 400 mg Tablet Oral<br />
1525 NOVAFEN PLUS<br />
IBUPROFEN+PARA<br />
CETAMOL<br />
400 mg+ 325<br />
mg Tablet Oral<br />
1526 NOVAFULVIN 125 GRISEOFULVIN 125 mg Tablet Oral<br />
1527 NOVAKOFF<br />
EUCALYPTUS<br />
OIL+MENTHOL+PE<br />
PPERMINT<br />
OIL+OTHERS<br />
30 mg/10<br />
mg/125<br />
mcg/100<br />
mcg/50<br />
mcg/25 mcg Tablet Oral<br />
1528 NOVATEN ATENOLOL 100 mg Tablet Oral<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
PHARMANOV<br />
A LTD.<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
EGYPTIAN<br />
INTERNATIONAL<br />
PHARMACEUTICAL<br />
IND. CO.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
PHARMANOVA<br />
LTD.<br />
PHARMANOVA<br />
LTD.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
PHARMANOVA<br />
LTD. 11/1/2011<br />
PHARMANOVA<br />
LTD. 8/1/2012<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
PHARMANOVA<br />
LTD. 4/1/2011<br />
PHARMANOVA<br />
LTD. 2/1/2013<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
178<br />
MARCH 1, 2011
1529 NOVATEN ATENOLOL 50 mg Tablet Oral<br />
1530 NOVATEN AM<br />
ATENOLOL+AMLO<br />
DIPINE 50 mg/5 mg Tablet Oral<br />
1531 NOVOTONE MULTIVITAMIN Multiple Tablet Oral<br />
1532<br />
1533<br />
NS SODIUM CHLORIDE<br />
I.V.<br />
NUCLEO C.M.P.<br />
FORTE<br />
1534 NUGEL<br />
1535 NUGEL-O<br />
1536 NUROVIT<br />
SODIUM<br />
CHLORIDE 0.9% w/v<br />
CMP DISODIUM +<br />
UDP DISODIUM 5mg/3mg<br />
ALGINIC<br />
ACID+ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
TRISILICATE Multiple<br />
ALGINIC<br />
ACID+ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
TRISILICATE Multiple<br />
VITAMIN B-<br />
COMPLEX+VITAMI<br />
N B12 Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
Injectable<br />
Solution<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
SRAVANI<br />
LABS LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
FERRER<br />
INTERNACION<br />
AL S.A<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
SRAVANI LABS<br />
LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
FERRER<br />
INTERNACIONAL<br />
S.A<br />
SAI MIRRA PHARM<br />
PRIVATE LIMITED<br />
SAI MIRRA PHARM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
D. K. D.<br />
PHARMACY LTD 8/1/2013<br />
SUPRA PHARMA<br />
LIMITED 11/1/2012<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 12/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 3/1/2013<br />
ROXIN GHANA<br />
LTD 10/1/2012<br />
179<br />
MARCH 1, 2011
1537 OCUMYCIN<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
CHLORAMPHENIC<br />
OL 1.0%w/w Eye ointment<br />
1538 OCUTIM TIMOLOL 0.5 %w/v Eye drops<br />
CENTAUR<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
CENTAUR<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIPLA<br />
LIMITED CIPLA LIMITED<br />
1539 OFTALAR PRANOPROFEN 0.1% Eye drops ALCON CUSI ALCON CUSI<br />
1540 OILATUM<br />
1541 OILATUM EMOLLIENT<br />
1542 OILATUM JUNIOR<br />
LIGHT LIQUID<br />
PARAFFIN +<br />
WHITE SOFT<br />
PARAFIN<br />
6%w/w +<br />
15%w/w<br />
LIGHT LIQUID<br />
PARAFFIN 63.4%w/w<br />
LIGHT LIQUID<br />
PARAFFIN +<br />
WHITE SOFT<br />
PARAFIN<br />
1543 OKA-CEFTRIAXONE CEFTRIAXONE 1 g<br />
1544 OKACID-20 OMEPRAZOLE 20 mg<br />
Cream<br />
Topical<br />
Topical<br />
Lotion<br />
Cream<br />
Topical<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S (IRELAND) LABORATORIES<br />
LTD (IRELAND) LTD<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S(UK) LABORATORIES<br />
LIMITED (IRELAND) LTD<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S(UK) LABORATORIES<br />
LIMITED (IRELAND) LTD<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
SUPRA PHARMA<br />
LIMITED 8/1/2013<br />
HEALTHCARE<br />
SERVICES LTD 11/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2011<br />
PARKERSTEIN<br />
(GH) LTD 3/1/2012<br />
PARKERSTEIN<br />
(GH) LTD 3/1/2012<br />
PARKERSTEIN<br />
(GH) LTD 3/1/2012<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
180<br />
MARCH 1, 2011
1545 OKADOL PARACETAMOL 125 mg Suppository<br />
1546 OKA-NEFIDIPINE NIFEDIPINE 20 mg Tablet Oral<br />
1547 OKA-OMEPRAZOLE OMEPRAZOLE 40 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
1548 OKAPRESS-50 ATENOLOL 50 mg Tablet Oral<br />
1549 OKARAN-100 DICLOFENAC 100 mg Suppository<br />
1550 OKA-RANITIDINE RANITIDINE 150 mg Tablet Oral<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OSAKA<br />
PHARMA PVT. OKASA PHARMA<br />
LTD. PVT LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA<br />
PHARMA PVT<br />
LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
OKASA PHARMA<br />
PVT LIMITED<br />
1551 OLFEN GEL DICLOFENAC 1%w/w Gel Topical MEPHA LTD. MEPHA LTD.<br />
1552 OLFEN LACTAB DICLOFENAC 50 mg Tablet Oral MEPHA LTD. MEPHA LTD.<br />
1553 OLFEN RECTOCAPS DICLOFENAC 100mg Suppository<br />
1554 OLFEN SR DEPOCAPS DICLOFENAC 100mg<br />
1555 OLFEN SR Depotabs DICLOFENAC 75 mg<br />
Capsule<br />
Extended<br />
Release<br />
Tablet<br />
Extended<br />
Realese<br />
PHARMA INFO<br />
CONSULT MEPHA LTD.<br />
PHARMA INFO<br />
CONSULT MEPHA LTD.<br />
PHARMA INFO<br />
CONSULT MEPHA LTD.<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 5/1/2012<br />
SUPRA PHARMA<br />
LIMITED 6/1/2012<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
SUPRA PHARMA<br />
LIMITED 6/1/2012<br />
SUPRA PHARMA<br />
LIMITED 10/1/2013<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
PHARMA INFO<br />
CONSULT 3/1/2013<br />
PHARMA INFO<br />
CONSULT 2/1/2013<br />
181<br />
MARCH 1, 2011
1556 OMEGARD OMEPRAZOLE 20 mg<br />
1557 OMEGA-T<br />
CAMPHOR+METHL<br />
Y<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
1558 OMEPRAZOLE OMEPRAZOLE 20 mg<br />
1559 OMEPRAZOLE OMEPRAZOLE 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Cream<br />
Topical<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1560 OMEROL PARACETAMOL 120 mg/5ml Oral Solution<br />
1561 OMERTONE TONIC<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup<br />
1562 OMEZ OMEPRAZOLE 40mg/ ml<br />
1563 OMIZAC OMEPRAZOLE 20 mg<br />
1564 ONOCID OMEPRAZOLE 20 mg<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
OKORMANSA-<br />
AMOA LTD<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
OMER<br />
INVESTMENTS<br />
LIMITED<br />
OMER<br />
INVESTMENTS<br />
LIMITED<br />
OKORMANSA-AMOA<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
OMER<br />
INVESTMENTS<br />
LIMITED<br />
OMER<br />
INVESTMENTS<br />
LIMITED<br />
DR REDDY'S DR REDDY'S<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
BIG MARON<br />
PHARMACY<br />
SALOM<br />
PHARMACY<br />
LIMITED 3/1/2013<br />
OKORMANSA-<br />
AMOA LTD 4/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
OMER<br />
INVESTMENTS<br />
LIMITED 11/1/2011<br />
OMER<br />
INVESTMENTS<br />
LIMITED 3/1/2012<br />
DR REDDY'S<br />
LABORATORIES<br />
LIMITED 5/1/2013<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 6/1/2013<br />
LARK<br />
LABORATORIES<br />
LTD<br />
BIG MARON<br />
PHARMACY 9/1/2012<br />
182<br />
MARCH 1, 2011
1565 OPIZOLE OMEPRAZOLE 20 mg<br />
1566 OPTICROM<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
SODIUM<br />
CROMOGLYCATE 2%w/v Eye drops<br />
1567 OPTIFENAC-50 DICLOFENAC 50 mg Tablet Oral<br />
1568 ORACIP TZ<br />
1569 ORADEX FORTE<br />
1570<br />
ORAL REHYDRATION<br />
SALT<br />
1571 ORALCON-F<br />
CIPROFLOXACIN+<br />
TINIDAZOLE<br />
500 mg/600<br />
mg Tablet Oral<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12 Multiple Syrup<br />
ORAL<br />
REHYDRATION<br />
SALT Multiple<br />
ETHINYLESTRADIO<br />
L+IRON+LEVONOR<br />
GESTREL<br />
1572 ORELOX CEFPODOXIME 100 mg<br />
1573 ORELOX PAEDIATRIC CEFPODOXIME 40 mg/5 mls<br />
1574 ORFIX CEFIXIME 200 mg<br />
0.15 mg/0.03<br />
mg/75 mg Tablet Oral<br />
GB PHARMA<br />
(uk) LTD.<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
NETAB<br />
PHARMACY<br />
SANKA (UK)<br />
LIMITED<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 7/1/2013<br />
AJANTA PHARMA<br />
(INDIA) LTD.<br />
MERCURY<br />
LABORATORIES<br />
LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
NETAB<br />
PHARMACY 7/1/2011<br />
SHREECHEM<br />
LABORATORIES PASS PHARMACY 9/1/2011<br />
Powder for<br />
Oral Solution DANNEX LTD DANNEX LTD DANNEX LTD 10/4/2014<br />
Tablet film<br />
coated<br />
Powder for<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
FAMY CARE<br />
LIMITED<br />
LABORATOIRE<br />
S AVENTIS<br />
LABORATOIRE<br />
S AVENTIS<br />
ORCHID<br />
HEALTHCARE<br />
FAMY CARE<br />
LIMITED<br />
GSMF<br />
INTERNATIONAL 6/1/2013<br />
USIPHAR<br />
(FRANCE) GOKALS LTD 8/1/2013<br />
USIPHAR<br />
(FRANCE) GOKALS LTD 8/1/2013<br />
ORCHID<br />
HEALTHCARE<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 4/1/2013<br />
183<br />
MARCH 1, 2011
1575 OROFER<br />
1576 OROFER FOLIC ACID+IRON<br />
IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX 50 mg/5ml Syrup<br />
100 mg/550<br />
mcg<br />
1577 ORZID CEFTAZIDIME 1000 mg<br />
1578 OSONMET METFORMIN 500 mg<br />
1579 OSON'S COD LIVER OIL COD LIVER OIL 300 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Tablet film<br />
coated<br />
1580 OSON'S WORMS PIPERAZINE 750 mg/5 ml Syrup<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1581 OTRIVIN XYLOMETAZOLINE 1% Nasal Spray<br />
1582 OTRIVIN XYLOMETAZOLINE 0.5 %v/v Nasal Drops<br />
1583 OTRIVIN XYLOMETAZOLINE 1% w/w Nasal Drops<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
ORCHID<br />
HEALTHCARE<br />
OSON'S<br />
CHEMIST LTD<br />
OSON'S<br />
CHEMIST LTD<br />
OSON'S<br />
CHEMIST LTD<br />
BETA<br />
HEALTHCARE<br />
BETA<br />
HEALTHCARE<br />
BETA<br />
HEALTHCARE<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 5/1/2013<br />
ORCHID<br />
HEALTHCARE<br />
MISSION VIVACARE<br />
LIMITED<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 12/31/2011<br />
OSON'S CHEMIST<br />
LTD 12/31/2012<br />
OSON'S CHEMIST<br />
LTD 2/1/2012<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
184<br />
MARCH 1, 2011
1584 OTRIVIN XYLOMETAZOLINE 1%w/w Gel Topical<br />
1585 OVIS ALBENDAZOLE 400 mg Tablet Oral<br />
1586 OXAFEN PIROXICAM 20 mg<br />
1587 OXYNIC<br />
1588 OXYNIC<br />
1589 OXYNIC<br />
1590 OXYNIC<br />
1591 OXYPOL<br />
1592 OXYTETRACYCLINE<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
OXYTETRACYCLIN<br />
E + POLYMYCIN<br />
500 mg+100<br />
mg<br />
500 mg+ 125<br />
mg<br />
400 mg+<br />
57mg<br />
250 mg+ 62.5<br />
mg<br />
OXYTETRACYCLIN<br />
E 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Tablet film<br />
coated<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
CIBA-GEIGY<br />
AG CIBA-GEIGY AG<br />
Y & J<br />
PHARMACEUT<br />
ICALS<br />
COMPANY<br />
LIMITED DAE HWA PHARM.<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
EGYPTIAN<br />
INTERNATION<br />
AL<br />
5 mg +10,<br />
PHARMACEUT<br />
000 IU Eye ointment ICAL IND. CO.<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
LINCOLN<br />
PHARMACEUTICALS<br />
LIMITED<br />
CASTOR & POLLUX<br />
PRIVATE LIMITED<br />
CASTOR & POLLUX<br />
PRIVATE LIMITED<br />
CASTOR & POLLUX<br />
PRIVATE LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
RIGHTVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
Y & J<br />
PHARMACEUTIC<br />
ALS COMPANY<br />
LIMITED 5/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
GB PHARMA<br />
(GH) LTD 9/1/2013<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
185<br />
MARCH 1, 2011
1593 OXYTETRACYCLINE<br />
OXYTETRACYCLIN<br />
E 250 mg<br />
1594 OXYTOCIN OXYTOCIN 10.00 iu/ml<br />
1595 OXYTOCIN OXYTOCIN 10 mg<br />
1596 PAIN HEAT<br />
BENZYL<br />
ALCOHOL+DICLOF<br />
ENAC+LINSEED<br />
OIL+MENTHOL+ME<br />
THYL SALICYL<br />
1.000 %<br />
w/w+1.160<br />
%w/w+3.000<br />
%w/w+5.000<br />
%w/w+10.00<br />
0%<br />
1597 PAINGAY DICLOFENAC 100 mg<br />
1598 PAINGAY<br />
1599 PAIN-OFF<br />
1600 PAINZAP<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME<br />
THYL SALICYLATE<br />
CAFFEINE+PARAC<br />
ETAMOL<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Ointment<br />
Topical<br />
Capsule<br />
Extended<br />
Release<br />
1% w/w/3%<br />
w/w//5%<br />
w/w/10%<br />
w/w Gel Topical<br />
500 mg, 65<br />
mg Tablet Oral<br />
30 mg+400<br />
mg+ 500 mg Tablet Oral<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
VAST FAVOUR<br />
INTERNATIONAL<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
SOCOMED PHARMA<br />
PVT LTD<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
GR<br />
INDUSTRIES<br />
LTD.<br />
STARWIN<br />
PRODUCTS LTD.<br />
GR INDUSTRIES<br />
LTD.<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
BEDITA<br />
PHARMACY 6/1/2013<br />
SALOM<br />
PHARMACY<br />
LIMITED 9/1/2012<br />
SALOM<br />
PHARMACY<br />
LIMITED 9/1/2012<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2011<br />
GR INDUSTRIES<br />
LTD. 7/1/2011<br />
186<br />
MARCH 1, 2011
1601 PAKARA<br />
1602 PALIDAR<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL Multiple Tablet Oral<br />
PYRIMETHAMINE+<br />
SULPHADOXINE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg/25<br />
mg Tablet Oral<br />
1603 PALUDRINE PROGUANIL 100 mg Tablet Oral<br />
1604 PAMPAC<br />
CAFFEINE+EPHED<br />
RINE<br />
HCL+PARACETAM<br />
OL<br />
30 mg+15<br />
mg+ 500 mg Tablet Oral<br />
1605 PANACIN PARACETAMOL 500 mg Tablet Oral<br />
1606<br />
PANCURONIUM<br />
BROMIDE-FRESENIUS PANCURONIUM 2 mg/1 ml<br />
1607 PARABRU<br />
Injectable<br />
Suspension<br />
IBUPROFEN+PARA<br />
CETAMOL 725 mg Tablet Oral<br />
1608 PARACETAMOL PARACETAMOL 500 mg Suppository<br />
1609 PARACETAMOL PARACETAMOL 1g Suppository<br />
MERCURY<br />
HEALTHCARE<br />
PVT. LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
PAM<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
AGOG PHARMA<br />
LTD.<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
NYCOMED GmbH<br />
GERMANY<br />
PAM<br />
PHARMACEUTICALS<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
PARMARTS<br />
PHARMACY LTD 1/13/2012<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/20/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2012<br />
PAM<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 3/1/2012<br />
FRESENIUS<br />
KABI (PTY)<br />
LTD (SOUTH<br />
AFRICA) AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 5/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
187<br />
MARCH 1, 2011
1610 PARACETAMOL PARACETAMOL 125 mg Suppository<br />
1611 PARACETAMOL PARACETAMOL 250 mg Suppository<br />
1612 PARACETAMOL PARACETAMOL 500 mg Tablet Oral<br />
1613 PARACETAMOL PARACETAMOL 500 mg Tablet Oral<br />
1614 PARACETAMOL PARACETAMOL 120 mgl5ml Elixir<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
PERFECT<br />
PHARMACEUT<br />
ICALS LTD<br />
PHARMANOV<br />
A LTD.<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
PERFECT<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
1615 PARACETAMOL BP PARACETAMOL 500 mg Tablet Oral NAMCO LTD. NAMCO LTD. NAMCO LTD. 10/1/2011<br />
1616 PARACETAMOL DENK PARACETAMOL 125 mg Suppository<br />
1617 PARACETAMOL DENK PARACETAMOL 250 mg Suppository<br />
1618 PARAFENAC<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
50 mg+ 500<br />
mg Tablet Oral<br />
1619 PARAKING PARACETAMOL 120 mgl5ml Syrup<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
RUBIEPHARM<br />
ARZNEIMITTEL<br />
GmbH<br />
RUBIEPHARM<br />
ARZNEIMITTEL<br />
GmbH<br />
BAADER SCHULZ<br />
LABORATORIES<br />
STARWIN<br />
PRODUCTS LTD.<br />
GOKALS-<br />
LABOREX LTD 11/1/2012<br />
GOKALS-<br />
LABOREX LTD 11/1/2012<br />
ROXIN GHANA<br />
LTD 10/1/2013<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2011<br />
188<br />
MARCH 1, 2011
1620 PARAKING PARACETAMOL 500 mg Tablet Oral<br />
1621 PARALEX-D<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
1622 PARAMOL PARACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
500 mg/50<br />
mg Tablet Oral<br />
1623 PARLODEL BROMOCRIPTINE 2.50 mg Tablet Oral<br />
1624 PASSIONS ZINC OXIDE<br />
1625 PATANOL<br />
1626<br />
PEACE KABA<br />
OINTMENT<br />
1627 PECTORAL<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
STARWIN<br />
PRODUCTS LTD. 4/1/2011<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 12/31/2012<br />
120 mgl5ml<br />
& 125ml/5ml Syrup DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2013<br />
Powder for<br />
topical use<br />
OLOPATADINE<br />
HYDROCHLORIDE 0.1%w/v Eye drops<br />
EUCALYPTUS+MEN<br />
THOL Multiple<br />
Ointment<br />
Topical<br />
NOVARTIS<br />
PHARMA AG<br />
CYBELE<br />
COSMETICS<br />
LTD<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
CHAMPION<br />
PEACE<br />
OINTMENT<br />
NOVARTIS FARMA<br />
SPA (ITALY)<br />
CYBELE COSMETICS<br />
LTD (NIGERIA)<br />
ALCON-<br />
COUVREUR<br />
CHAMPION PEACE<br />
OINTMENT<br />
PLANTAGINIS+PRI<br />
MULAE+SENEGAE+<br />
THYME MULTIPLE Syrup MEPHA LTD. MEPHA LTD.<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
CYBELE<br />
COSMETICS LTD<br />
(NIGERIA) 4/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 4/1/2011<br />
CHAMPION<br />
PEACE<br />
OINTMENT 12/1/2011<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
189<br />
MARCH 1, 2011
1628 PEGASYS<br />
1629 PEG-INTRON<br />
1630 PEG-INTRON<br />
1631 PEG-INTRON<br />
1632 PEG-INTRON<br />
1633 PEG-INTRON<br />
PEGINTERFERON<br />
ALFA-2A 180 mcg/ml<br />
PEG INTERFERON<br />
ALPHA 2B 100 mcg/vial<br />
PEG INTERFERON<br />
ALPHA 2B 120 mcg/vial<br />
PEG INTERFERON<br />
ALPHA 2B 50 mcg/vial<br />
PEG INTERFERON<br />
ALPHA 2B 80 mcg/vial<br />
PEG INTERFERON<br />
ALPHA 2B 150 mcg/vial<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
F. HOFFMANN-LA<br />
ROCHE<br />
LTD.(Switzerland)<br />
F. HOFFMAN-LA<br />
ROCHE INC(USA)<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 12/1/2012<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND) GOKALS LTD 7/1/2012<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND) GOKALS LTD 7/1/2012<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND) GOKALS LTD 7/1/2012<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND) GOKALS LTD 7/1/2012<br />
SCHERING PLOUGH<br />
(BRINNY) CO<br />
(IRELAND) GOKALS LTD 7/1/2012<br />
190<br />
MARCH 1, 2011
1634 PENGESIC TRAMADOL 50 mg<br />
1635 PENICILLIN V PENICILLIN V 125 mg Tablet Oral<br />
1636 PENICILLIN V.K. PENICILLIN V 125 mg Tablet Oral<br />
1637 PERFECT'S LIVER SALT<br />
CITRIC<br />
ACID+MAGNESIUM<br />
SULPHATE+SODIU<br />
M BICARBONATE<br />
35.65% +<br />
18.61% +<br />
45.54%<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin) HOVID Bhd. HOVID Bhd.<br />
Powder for<br />
Oral Solution<br />
1638 PERSANTIN DIPYRIDAMOLE 2.5 mg Tablet Oral<br />
1639<br />
PETHIDINE HCL-<br />
Fresenius PF<br />
1640 PETOGEN-FRESENIUS<br />
PETHIDINE<br />
HYDROCHLORIDE 100 mg/ 2 ml<br />
MEDROXYPROGES<br />
TERONE 150 mg/ml<br />
1641 PEVARYL ECONAZOLE 10 mg/g<br />
Injectable<br />
Solution<br />
Injectable<br />
Suspension<br />
Cream<br />
Topical<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
PERFECT<br />
PHARMACEUT<br />
ICALS LTD<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
HELM<br />
PHARMACEUT<br />
ICALS GmbH<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
PERFECT<br />
PHARMACEUTICALS<br />
LTD<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
BEDITA<br />
PHARMACY 9/1/2013<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 12/31/2012<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
PERFECT<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 10/4/2013<br />
HILLS<br />
PHARMACY 7/1/2013<br />
SIMUMEERA<br />
PHARMACY LTD. 10/1/2012<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
191<br />
MARCH 1, 2011
1642 PEVISONE<br />
1643<br />
1644<br />
1645<br />
PHARMADERM CARE<br />
TALC<br />
PHARMADERM<br />
PURIFYING BODY<br />
ECONAZOLE+TRIA<br />
MCINOLONE 10 mg/g<br />
BENZOIC<br />
ACID+SALICYLIC<br />
ACID+SULPHUR Multiple<br />
BENZOIC<br />
ACID+SALICYLIC<br />
ACID+SULPHUR+ZI<br />
NC OXIDE Multiple<br />
PHARMADERM<br />
PURIFYING LOTION SULPHUR Multiple<br />
1646 PHARMADERM SOAP<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Powder for<br />
topical use<br />
Topical<br />
Lotion<br />
Topical<br />
Lotion<br />
BENZOIC<br />
ACID+SALICYLIC<br />
ACID+SULPHUR Multiple Solid Soap<br />
1647 PHARMAGENT GENTAMICIN 40 mg/ml<br />
1648<br />
PHARMANOVA<br />
MULTIVITAMIN MULTIVITAMIN Multiple<br />
1649 PHARMATOCIN OXYTOCIN 10.00 iu/ml<br />
Injectable<br />
Solution<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
PHARMANOV<br />
A LTD.<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR CL<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR (GH)<br />
LTD. 3/1/2012<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR (GH)<br />
LTD. 3/1/2012<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR (GH)<br />
LTD. 3/1/2012<br />
NOUVELLE<br />
PARFUMERIE<br />
GANDOUR (GH)<br />
LTD. 2/16/2012<br />
OSON'S CHEMIST<br />
LTD 6/1/2012<br />
PHARMANOVA<br />
LTD. 8/1/2012<br />
OSON'S CHEMIST<br />
LTD 9/1/2011<br />
192<br />
MARCH 1, 2011
1650 PHARMAZINE<br />
PROMETHAZINE<br />
HYDROCHLORIDE 50 mg/2 ml<br />
1651 PHARMIDE FRUSEMIDE 20 mg/2 mls<br />
1652 PHARMONATE - 400<br />
AMODIAQUINE+AR<br />
TESUNATE<br />
300 mg/100<br />
mg<br />
1653 PHARNALGIN METAMIZOLE 2.5mg/5 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
1654 PHENOBARBITONE PHENOBARBITONE 60 mg Tablet Oral<br />
1655 PHENOBARBITONE PHENOBARBITONE 200 mg/ml<br />
Injectable<br />
Solution<br />
1656 PHENOBARBITONE PHENOBARBITONE 30 mg Tablet Oral<br />
1657 PHENTOLAMINE PHENTOLAMINE 10 mg/ml<br />
1658 PHENYLEPHRINE PHENYLEPHRINE 10 mg<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
1659 PHENYTOIN PHENYTOIN 100 mg Tablet Oral<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
PHARMANOV<br />
A LTD.<br />
SHANGHAI<br />
PHARMATEQ<br />
CO. LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
YANZHOU XI'ER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
COMPANY<br />
ERNEST CHEMISTS<br />
LIMITED<br />
OSON'S CHEMIST<br />
LTD 9/1/2011<br />
OSON'S CHEMIST<br />
LTD 9/1/2011<br />
PHARMANOVA<br />
LTD. 10/1/2012<br />
OSON'S CHEMIST<br />
LTD 9/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
ROCK<br />
CHEMISTS DANNEX LTD ROCK CHEMISTS 2/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
ROCK<br />
CHEMISTS<br />
ERNEST CHEMISTS<br />
LIMITED<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 3/24/2014<br />
UNICOM<br />
CHEMIST<br />
LIMITED 3/24/2014<br />
SUBVICK<br />
CHEMICALS, UK ROCK CHEMISTS 11/1/2011<br />
193<br />
MARCH 1, 2011
1660 PHLEBODIA DIOSMIN 600 mg<br />
1661 PHOPAIN<br />
1662<br />
PHTHALYLSULPHATHI<br />
AZOLE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL Multiple Tablet Oral<br />
PHTHALYLSULPHA<br />
THIAZOLE 500 mg Tablet Oral<br />
1663 PHYTOMOX AMOXICILLIN 125 mg/5 mls<br />
1664<br />
PICANN TEETHING<br />
REMEDY<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL<br />
Powder for<br />
Oral<br />
Suspension<br />
120 mg/125<br />
mg/5 mls Syrup<br />
1665 PILOCARPINE PILOCARPINE 2% w/v Eye drops<br />
1666 PILOCARPINE PILOCARPINE 4% w/v Eye drops<br />
1667 PILOCARPINE PILOCARPINE 4% w/v Eye drops<br />
LABORATOIRE<br />
INNOTECH<br />
INTERNATION<br />
AL<br />
TRADE WINDS<br />
CHEMIST LTD<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
KENSINGTON<br />
PHARMACEUT<br />
ICALS<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
INNOTHERA<br />
CHOUZY<br />
(INNOTHERA<br />
GROUP)<br />
TRADE WINDS<br />
CHEMIST LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
KENSINGTON<br />
PHARMACEUTICALS<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
TRADE WINDS<br />
CHEMIST LTD 8/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 7/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
194<br />
MARCH 1, 2011
1668 PINKOO GRIPE WATER<br />
1669 PIRIKIN<br />
1670 PIRITEXYL-BR<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
ANISE+BICARBONA<br />
TE+DILL+MENTHO<br />
L Multiple Oral Solution<br />
CHLORPHENIRAMI<br />
NE 4 mg Tablet Oral<br />
AMBROXOL+TERB 15 mg/1.25<br />
UTALINE mg/50<br />
+GUAIFENESIN+ME mg/2.5 mg/5<br />
NTHOL<br />
mls Syrup<br />
1671 PIROXIKIN PIROXICAM 20mg<br />
1672 PIZAVIT<br />
1673<br />
1674<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PIZOTIFEN+VITAM<br />
INS Multiple Syrup<br />
PLASMOTRIM - 200<br />
RECTO CAPS ARTESUNATE 200 mg<br />
AJANTA<br />
PHARMA<br />
(INDIA) LTD.<br />
AJANTA PHARMA<br />
(INDIA) LTD.<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
JENBURKT<br />
PHARMACEUT<br />
ICALS LTD<br />
Capsule oral<br />
(Hard<br />
gelatin) MEPHA LTD.<br />
JENBURKT<br />
PHARMACEUTICALS<br />
LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
MULTINATION<br />
AL BUSINESS<br />
LINK ALINA COMBINE<br />
MEPHA<br />
LTD.MEPHA LTD.<br />
PLASMOTRIM -<br />
RECTOCAP ARTESUNATE 50 mg Tablet Oral MEPHA LTD. MEPHA LTD.<br />
1675 PLAVIX CLOPIDOGREL 75 mg<br />
1676 PLEMETHER ARTEMETHER 80 mg/ml<br />
1677 PLENDIL FELODIPINE 10 mg<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
Tablet<br />
Extended<br />
Realese<br />
SANOFI<br />
AVENTIS<br />
FRANCE<br />
PLETHICO<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
ZETA<br />
PHARMACY LTD 7/1/2011<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
SALOM<br />
PHARMACY<br />
LIMITED 10/1/2011<br />
PHARMA INFO<br />
CONSULT 9/1/2013<br />
PHARMA INFO<br />
CONSULT 9/1/2013<br />
SANOFI<br />
WINTHROP(France) GOKALS-<br />
LABOREX LTD 9/1/2012<br />
PLETHICO<br />
PHARMACEUTICALS<br />
LTD<br />
ASTRAZENECA<br />
AB(Sweden)<br />
PHARMANOVA<br />
LTD. 5/10/2013<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
195<br />
MARCH 1, 2011
1678 PLETROX-1000 CEFTRIAXONE 1g<br />
1679<br />
PLUS MULTIVITAMIN &<br />
MINERAL<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
1680 PMF CLASSIC PARAFFIN 100% w/w<br />
1681<br />
POLAR ESSENTIAL<br />
EMBROCATION<br />
1682 POLYGEL<br />
MENTHOL+PEPPER<br />
MINT+OTHERS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PLETHICO<br />
PHARMACEUT<br />
ICALS LTD<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED<br />
(INDIA)<br />
PLETHICO<br />
PHARMACEUTICALS<br />
LTD<br />
MEGA<br />
LIFESCIENCES<br />
LIMITED (INDIA)<br />
PHARMANOVA<br />
LTD. 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 10/1/2011<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 2/1/2013<br />
Topical<br />
solution<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS Multiple Tablet Oral<br />
1683 PONSTAN MEFENAMIC ACID 250 mg<br />
1684 PONTALON MEFENAMIC ACID 250 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1685 PORO 125 ACETAMINOPHEN 125 mg Suppository<br />
SHANGHAI<br />
MEDICINES &<br />
HEALTH<br />
PRODUCTS<br />
CORP<br />
SHANGHAI<br />
MEDICINES &<br />
HEALTH PRODUCTS<br />
CORP<br />
SHALINA<br />
LABORATORIE<br />
S PVT. LTD. SHALINA JEJURI<br />
PARKE-DAVIS<br />
(SENEGAL)<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
PARKE-DAVIS<br />
(SENEGAL)<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
PHILAQUAYE<br />
MILLIONAIRE<br />
CO. LTD 9/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 8/18/2013<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 8/1/2012<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
196<br />
MARCH 1, 2011
1686 PORO 250 ACETAMINOPHEN 250 mg Suppository<br />
1687 POTASSIUM CHLORIDE<br />
1688 POTASSIUM CHLORIDE<br />
1689<br />
POTASSIUM<br />
PERMANGANATE<br />
POTASSIUM<br />
CHLORIDE 1 g<br />
POTASSIUM<br />
CHLORIDE 10%<br />
POTASSIUM<br />
PERMANGANATE 99%w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Powder for<br />
Topical<br />
Solution<br />
1690 POVIDONE IODINE POVIDONE IODINE 1%w/v Oral Solution<br />
1691 POWERGESIC PLUS<br />
DICLOFENAC+MEN<br />
THOL+METHYL<br />
SALICYLATE<br />
1.16<br />
%w/w/10<br />
%w/w/ 5<br />
%w/w Gel Topical<br />
1692 PRAZIQUANTEL PRAZIQUANTEL 600 mg Tablet Oral<br />
1693 PRAZOLIN PANTOPRAZOLE 20 mg<br />
1694 PRAZOLIN PANTOPRAZOLE 40 mg<br />
Tablet<br />
Enteric<br />
Coated<br />
Tablet<br />
Enteric<br />
Coated<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD.<br />
KAMA INDUSTRIES<br />
LTD.<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD MEDPHARMA<br />
JENBURKT<br />
PHARMACEUT<br />
ICALS LTD<br />
JENBURKT<br />
PHARMACEUTICALS<br />
LTD<br />
IDA<br />
FOUNDATION CIPLA LIMITED<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
SANDOZ ILAC<br />
SANAYI (TURKEY)<br />
SANDOZ ILAC<br />
SANAYI (TURKEY)<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 9/23/2011<br />
HEALTH ACCESS<br />
NETWORK 4/1/2012<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
197<br />
MARCH 1, 2011
1695 PRED FORTE PREDNISOLONE 10 mg/ ml Eye drops<br />
1696 PREDNISOLONE PREDNISOLONE 5 mg Tablet Oral<br />
1697 PREGNACARE<br />
1698 PREGRON-M<br />
1699 PRESARTAN H 50<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
FOLIC<br />
ACID+IRON+SELEN<br />
IUM+VITAMIN<br />
B12+VITAMIN E<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1 mg/60<br />
mg/60 mcg/5 Capsule oral<br />
mcg/15 iu (Softgel)<br />
HYDROCHLOROTH<br />
IAZIDE+LOSARTAN 50/12.5 mg Tablet Oral<br />
1700 PRESATAN-100 LOSARTAN 100 mg<br />
Tablet film<br />
coated<br />
1701 PRIMADOL PARACETAMOL 500 mg Tablet Oral<br />
1702 PRIMADOL PARACETAMOL 120 mg/5 ml Syrup<br />
1703 PRIMOLUT DEPO<br />
HYDROXYPROGES<br />
TERONE 250 mg/ml<br />
Injectable<br />
Solution<br />
1704 PRIMOLUT N NORETHISTERONE 5 mg Tablet Oral<br />
ALLERGAN<br />
PHARMACEUT<br />
ICALS<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
MEYER<br />
ORGANICS<br />
LTD.<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
ALLERGAN<br />
PHARMACEUTICALS BERNSWETT<br />
COMPANY LTD 6/1/2011<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
MEYER HEALTH<br />
CARE PVT LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BAYER - SCHERING<br />
PHARMA<br />
BAYER - SCHERING<br />
PHARMA<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
GR PHARMA<br />
LTD. 10/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
198<br />
MARCH 1, 2011
1705 PROACTIV<br />
1706 PROCAINE PENICILLIN<br />
1707 PROCAINE PENICILLIN<br />
CAFFEINE+EPHED<br />
RINE<br />
HCL+PARACETAM<br />
OL<br />
FORTIFIED<br />
PROCAINE<br />
PENICILLIN 4 MU<br />
FORTIFIED<br />
PROCAINE<br />
PENICILLIN 3 mega<br />
1708 PROCOMIL LIDOCAINE 10g/100ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg + 10<br />
mg + 500 mg Tablet Oral<br />
Injectable<br />
Solution<br />
Powder for<br />
Oral<br />
Suspension<br />
Topical<br />
Spray<br />
1709 PROCOMIL YOHIMBINE 5 mg Tablet Oral<br />
1710 PROCTO-GLYVENOL<br />
LIDOCAINE+TRIBE<br />
NOSIDE 5%, 2%w/w<br />
Cream<br />
Rectal<br />
1711 PROFLAX CIPROFLOXACIN 500 mg Tablet Oral<br />
1712 PROGYNOVA ESTRADIOL 2 mg Tablet Oral<br />
TOBINCO<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
OSAKA PHARMA<br />
PVT. LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ALEMBIC<br />
LIMITED ALEMBIC LIMITED<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
BETA<br />
HEALTHCARE<br />
GLAXOSMITH<br />
KLINE<br />
CONSUMER<br />
NIGERIA LTD<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
NOVARTIS<br />
CONSUMER<br />
HEALTHCARE S.A.<br />
MEDREICH<br />
LIMITED<br />
BAYER -<br />
SCHERING<br />
PHARMA BAYER - SCHERING<br />
AFRICA GMBH PHARMA<br />
TOBINCO<br />
PHARMACY 10/1/2012<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 10/3/2013<br />
DOVE<br />
PHARMACY<br />
LIMITED 9/15/2011<br />
UNICHEM<br />
GHANA LTD. 3/1/2012<br />
UNICHEM<br />
GHANA LTD. 4/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 12/31/2011<br />
BAYER -<br />
SCHERING<br />
PHARMA AFRICA<br />
GMBH 2/1/2013<br />
199<br />
MARCH 1, 2011
1713 PROMECINE<br />
1714 PROMETHAZINE<br />
1715 PROMETHAZINE<br />
1716 PROMETHAZINE<br />
1717 PROMIZEL<br />
PROMETHAZINE<br />
HYDROCHLORIDE 5 mg/5mls Syrup<br />
PROMETHAZINE<br />
HYDROCHLORIDE 25 mg/ ml<br />
PROMETHAZINE<br />
HYDROCHLORIDE 50 mg/ 2ml<br />
PROMETHAZINE<br />
HYDROCHLORIDE 25 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
PROMETHAZINE<br />
HYDROCHLORIDE 5 mg/5 mls Syrup<br />
1718 PROPOVAN PROPOFOL 1% w/w<br />
1719<br />
1720<br />
1721<br />
1722<br />
Injectable<br />
Solution<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 40 mg Tablet Oral<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 40 mg Tablet Oral<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 80 mg Tablet Oral<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 40 mg Tablet Oral<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
VAST FAVOUR<br />
INTERNATIONAL<br />
LTD<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/2/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 2/1/2012<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
PIRIMAL<br />
HEALTHCARE<br />
LIMITED<br />
(INDIA)<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 12/31/2012<br />
BHARAT SERUMS<br />
AND VACCINES<br />
LTD. PNT PHARMACY 12/21/2008<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
200<br />
MARCH 1, 2011
1723<br />
1724<br />
1725<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 80 mg Tablet Oral<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 40 mg Tablet Oral<br />
PROPRANOLOL<br />
HYDROCHLORIDE PROPRANOLOL 40 mg Tablet Oral<br />
1726 PROSCAR FINASTERIDE 5 mg Tablet Oral<br />
1727 PROSTAN LATANOPROST 0.05%w/v Eye drops<br />
1728 PROTAMIN PROTAMIN 5 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
1729 PROVIRON MESTEROLONE 25 mg Tablet Oral<br />
1730 PROVIVE PROPOFOL 1%w/v<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
SHANDONG<br />
XINGHUA<br />
PHARMACEUT<br />
ICAL CO.<br />
LTD.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
SHANDONG<br />
XINGHUA<br />
PHARMACEUTICAL<br />
CO. LTD.<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 5/1/2012<br />
UNICOM<br />
CHEMIST<br />
LIMITED 4/1/2012<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
BAYER - SCHERING<br />
PHARMA<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
BAYER -<br />
SCHERING<br />
Ghana office. 9/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2011<br />
201<br />
MARCH 1, 2011
1731 PROXIMEXA CEFUROXIME 125 mg/5ml<br />
1732 PROXIMEXA CEFUROXIME 250 mg/5ml<br />
1733 PROXIMEXA CEFUROXIME 250 mg<br />
1734 PROXIMEXA CEFUROXIME 500 mg<br />
1735 PULMICORT BUDESONIDE 0.25 mg/ml<br />
1736 PULMICORT BUDESONIDE 0.5 mg/ml<br />
1737<br />
PULMICORT<br />
TURBUHALER BUDESONIDE<br />
200<br />
mcg/dose<br />
1738 PULMOXYL AMOXICILLIN 250 mg<br />
1739 PYRADOX<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Powder for<br />
Oral<br />
Suspension<br />
Powder for<br />
Oral<br />
Suspension<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Inhalational<br />
solution<br />
Inhalational<br />
solution<br />
Inhalational<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
PYRIMETHAMINE+<br />
SULPHADOXINE 500/25 mg Tablet Oral<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
BROWN &<br />
BURK<br />
PHARMACEUT<br />
ICALS LTD.<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
GLAXOSMITHKLINE<br />
UK LIMITED<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 9/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 9/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2012<br />
MICRO<br />
EXPORTS(LABORAT<br />
ORIES) GOKALS LTD 6/1/2011<br />
MISSION VIVACARE<br />
LIMITED<br />
OSON'S CHEMIST<br />
LTD 7/1/2012<br />
202<br />
MARCH 1, 2011
1740 PYRAZINAMIDE PYRAZINAMIDE 400 mg<br />
1741 Q-PAREL<br />
1742 QUADRAJEL<br />
1743 QUADRIDERM<br />
1744 QUINIMAX<br />
1745 QUINIMAX<br />
1746 QUINIMAX<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
CHLORHEXIDINE+L<br />
IDOCAINE+METRO<br />
NIDAZOLE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
50 mg/650<br />
mg Tablet Oral<br />
1%w/w/2%w/<br />
w/1%w/w Gel Oral<br />
BETAMETHASONE<br />
+CLIOQUINOL+GE 0.5 mg/10<br />
NTAMYCIN+TOLNA mg/1 mg/10<br />
FTATE<br />
mg<br />
Cream<br />
Topical<br />
CINCHONIDINE+CI<br />
NCHONINE+QUINI<br />
DINE+QUININE 500 mg Tablet Oral<br />
CINCHONIDINE+CI<br />
NCHONINE+QUINI<br />
DINE+QUININE 125 mg Tablet Oral<br />
CINCHONIDINE+CI<br />
NCHONINE+QUINI<br />
DINE+QUININE 250 mg/2 ml<br />
1747 QUININE QUININE 300 mg/ml<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
SVIZERA<br />
EUROPE BV<br />
HEALTHILIFE<br />
PHARMA PVT.<br />
LTD(INDIA)<br />
SVIZERA LABS PVT.<br />
LTD<br />
HEALTHILIFE<br />
PHARMA PVT.<br />
LTD(INDIA)<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SANOFI<br />
WINTHROP<br />
LTD(UK)<br />
SANOFI<br />
WINTHROP<br />
LTD(UK)<br />
SANOFI<br />
WINTHROP<br />
LTD(UK)<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 5/1/2011<br />
TEAM<br />
PHARMACEUTIC<br />
ALS LTD. 5/1/2012<br />
GR PHARMA<br />
LTD. 8/1/2011<br />
SCHERING-PLOUGH<br />
S.A. (SPAIN) GOKALS LTD 7/1/2012<br />
SANOFI WINTHROP<br />
LTD(UK) REISS & CO. 8/1/2013<br />
SANOFI WINTHROP<br />
LTD(UK) REISS & CO. 8/1/2013<br />
SANOFI WINTHROP<br />
LTD(UK) REISS & CO. 9/1/2013<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTIC<br />
ALS PVT LTD 4/1/2013<br />
203<br />
MARCH 1, 2011
1748 QUININE SULPHATE QUININE 300mg<br />
1749 QUININE SULPHATE QUININE 300mg<br />
1750 QUININE SULPHATE QUININE 300mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
1751 QUININE SULPHATE QUININE 300 mg Tablet Oral<br />
1752 QUINIRON QUININE 300 mg<br />
1753 QUINTOR CIPROFLOXACIN 500 mg<br />
1754 QUINTOR CIPROFLOXACIN<br />
200 mg/100<br />
ml<br />
Injectable<br />
Solution<br />
Tablet film<br />
coated<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
1755 RABEMAC RABEPRAZOLE 20 mg Tablet Oral<br />
1756 RAIDAVIT<br />
MINERALS +<br />
VITAMINS Multiple Syrup<br />
UNICOM<br />
CHEMIST<br />
LIMITED IMPRES<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
CIRON <strong>DRUG</strong>S<br />
&<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
MACLEODS<br />
PHARMACEUT<br />
ICALS LTD<br />
POKU<br />
PHARMA LTD.<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 4/1/2013<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 2/1/2013<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 2/1/2013<br />
MACLEODS<br />
PHARMACEUTICALS<br />
LTD DARL PHARMA 6/10/2013<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
POKU PHARMA<br />
LTD. 8/1/2012<br />
204<br />
MARCH 1, 2011
1757 RAMIPRIL RAMIPRIL 5 mg Tablet Oral<br />
1758 RAMIPRIL RAMIPRIL 10 mg Tablet Oral<br />
1759 RANI-DENK RANITIDINE 150.00 mg Tablet Oral<br />
1760 RANITAL RANITIDINE 50 mg/2 mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
1761 RANITIDINE RANITIDINE 150 mg Tablet Oral<br />
1762 RANITIDINE RANITIDINE 300 mg Tablet Oral<br />
1763 RANITIDINE RANITIDINE 50 mg/2 mls<br />
Injectable<br />
Solution<br />
1764 RANITIDINE RANITIDINE 150 mg Tablet Oral<br />
1765 RANITIDINE RANITIDINE 300 mg Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
KIMI & OUBARI<br />
PHARMACEUTICALS<br />
CO. LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
GOKALS-<br />
LABOREX LTD 4/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/31/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
205<br />
MARCH 1, 2011
1766 RANITIDINE RANITIDINE 150 mg Tablet Oral<br />
1767 RANITIDINE RANITIDINE 300 mg Tablet Oral<br />
1768 R-DIMOL<br />
DICYCLOMINE+PA<br />
RACETAMOL<br />
1769 RECORMON EPOETINUM BETA 2000 iu/ml<br />
1770 RECORMON EPOETINUM BETA 4000 iu/ml<br />
1771 REDIN PLUS<br />
1772 REDIN PN LIQUID<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS MULTIPLE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
20 mg + 500<br />
mg Tablet Oral<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
AMINO<br />
ACIDS+FOLIC<br />
ACID+IRON+VITB.<br />
12 Multiple Oral Solution<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
R H R<br />
MEDICARE P.<br />
LTD<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
RHR MEDICARE P.<br />
LTD<br />
F. HOFFMAN LA-<br />
ROCHE PRODUCTS<br />
(PTY) LTD. (SOUTH<br />
AFRICA)<br />
F. HOFFMAN LA-<br />
ROCHE PRODUCTS<br />
(PTY) LTD. (SOUTH<br />
AFRICA)<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
HOVID<br />
PHARMACEUTIC<br />
ALS LTD 7/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
FAR EAST<br />
MERCANTILE 11/1/2012<br />
FAR EAST<br />
MERCANTILE 11/1/2012<br />
206<br />
MARCH 1, 2011
1773 RELAXYL SR TABLETS DICLOFENAC 100mg Tablet Oral<br />
1774 RELCER<br />
ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
HYDROXIDE+SIME<br />
THICONE+OTHERS Multiple<br />
1775 REMICADE INFLIXIMAB 100 mg/Vial<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
1776 RETARDINE XL NIFEDIPINE 30 mg Tablet Oral<br />
1777 RETIN A TRETINOIN 0.025%w/w Gel Topical<br />
1778 REVIT MULTIVITAMIN Multiple Tablet Oral<br />
PRINCEPS<br />
PHARMACEUT<br />
ICALS PVT<br />
.LTD.<br />
GLENMARK<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
KORLYNS<br />
PHARMACEUT<br />
ICALS INT<br />
LTD<br />
JANSSEN-<br />
CILAG<br />
(SWITZERLAN<br />
D)<br />
TOBINCO<br />
PHARMACY<br />
PRINCEPS<br />
PHARMACEUTICALS<br />
PVT .LTD.<br />
GLENMARK<br />
PHARMACEUTICALS<br />
LIMITED<br />
REGENT<br />
PHARMACY 12/1/2013<br />
BERNSWETT<br />
COMPANY LTD 6/1/2011<br />
PATHEON ITALIA<br />
SpASCHERING<br />
PLOUGH (BRINNY)<br />
CO<br />
(IRELAND)PARKDA<br />
LE<br />
PHARMACEUTICALS<br />
INC. GOKALS LTD 3/1/2012<br />
AKUM <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
LTD<br />
JANSSEN-CILAG<br />
(SWITZERLAND)<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 7/1/2012<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 8/1/2011<br />
TOBINCO<br />
PHARMACY 10/1/2012<br />
207<br />
MARCH 1, 2011
1779 RE-ZOT<br />
1780 RE-ZOT<br />
ALGINIC<br />
ACID+ALUMINIUM<br />
HYDROXIDE+MAG<br />
NESIUM<br />
TRISILICATE+OTH<br />
ER<br />
200 mg + 250<br />
mg + 250 mg<br />
/15 ml<br />
ALUMINIUM<br />
HYDROXIDE+DIME<br />
THICONE+MAGNES<br />
IUM<br />
250 mg + 60<br />
HYD+MAGNESIUM mg + 250 mg<br />
TRI<br />
+ 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Tablet<br />
Chewable<br />
1781 RHEUMA DENK ETOFENAMATE 100 mg/g Gel Topical<br />
1782 RHIZIN CETIRIZINE 5 mg/5 mls<br />
1783 RICHTOL<br />
1784<br />
RIDDLES COUGH<br />
SYRUP(ADULT)<br />
EUCALYPTUS+MEN<br />
THOL<br />
GUAIPHENESIN+DI<br />
PHENHYDRAMINE<br />
HCL+MENTHOL+S<br />
ODIUM CITRATE<br />
Oral<br />
Suspension<br />
0.005 g/0.01<br />
g Lozenges<br />
100mg/5ml +<br />
10mg/5ml+1.<br />
2mg/5ml+55<br />
mg/5ml Syrup<br />
POKU<br />
PHARMA LTD.<br />
POKU<br />
PHARMA LTD.<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
POKU PHARMA<br />
LTD. 9/1/2012<br />
POKU PHARMA<br />
LTD. 2/1/2013<br />
GOKALS-<br />
LABOREX LTD 12/31/2011<br />
KINAPHARMA<br />
LTD 3/1/2012<br />
GLOW VARUN LIFE<br />
PHARMA PVT. SCIENCES PVT.<br />
LIMITED LTD. GEO PHARMACY 5/1/2013<br />
TRADE WINDS<br />
CHEMIST LTD<br />
TRADE WINDS<br />
CHEMIST LTD<br />
POKU PHARMA<br />
LTD. 3/1/2013<br />
208<br />
MARCH 1, 2011
1785<br />
1786<br />
RIDDLES<br />
MULTIVITAMIN SYRUP MULTIVITAMIN Multiple Syrup<br />
RIFAMPICIN +<br />
ISONIAZIDE +<br />
PYRAZINAMIDE +<br />
ETHAMBUTOL<br />
1787 RIFAMPICIN ISONIAZID<br />
1788<br />
1789<br />
RIFAMPICIN,<br />
ISONIAZID,<br />
ETHAMBUTOL<br />
RIFAMPICIN,<br />
ISONIAZID,<br />
PYRAZINAMIDE,<br />
ETHAMBUTOL<br />
ETHAMBUTOL+ISO<br />
NIAZID+PYRAZINA<br />
MIDE+RIFAMPICIN<br />
ISONIAZID+RIFAM<br />
PICIN<br />
ETHAMBUTOL+ISO<br />
NIAZID+RIFAMPICI<br />
N<br />
ETHAMBUTOL+ISO<br />
NIAZID+PYRAZINA<br />
MIDE+RIFAMPICIN<br />
275 mg + 75<br />
mg + 400<br />
mg+150 mg<br />
150 mg/75<br />
mg<br />
150 mg/75<br />
mg/275 mg<br />
150 mg/75<br />
mg/400<br />
mg/275 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
1790 RIFER DROPS IRON 50 mg/ml Syrup<br />
1791 RINGERS LACTATE<br />
RINGER'S<br />
LACTATE Multiple<br />
Injectable<br />
Solution<br />
1792 RISPERDAL RISPERIDONE 2 mg Tablet Oral<br />
TRADE WINDS<br />
CHEMIST LTD<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
SVIZERA<br />
EUROPE BV<br />
SVIZERA<br />
EUROPE BV<br />
SVIZERA<br />
EUROPE BV<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
TRADE WINDS<br />
CHEMIST LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
SVIZERA LABS PVT.<br />
LTD<br />
SVIZERA LABS PVT.<br />
LTD<br />
SVIZERA LABS PVT.<br />
LTD<br />
XL LABORATORIES<br />
PVT LTD<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
JANSSEN-CILAG<br />
S.p.A.(ITALY)<br />
TRADE WINDS<br />
CHEMIST LTD 2/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 5/1/2013<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 5/24/2011<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 5/1/2011<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 5/1/2011<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 11/1/2012<br />
DS SPRING<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 10/1/2011<br />
209<br />
MARCH 1, 2011
1793 RITALIN<br />
1794 RITONIC<br />
METHYLPHENIDAT<br />
E 10 mg Tablet Oral<br />
FOLIC<br />
400 mcg+50<br />
ACID+IRON+VITAM mg+5 mcg+5<br />
IN B12+ZINC mg Syrup<br />
1795 RIZOLE METRONIDAZOLE 200 mg Tablet Oral<br />
1796<br />
RL-COMPOUND<br />
SODIUM LACTATE I.V.<br />
1797 ROBB<br />
1798 ROBB INHALER<br />
1799<br />
ROBB SUPER INTENSE<br />
HEAT<br />
COMPOUND<br />
SODIUM LACTATE Multiple<br />
CAMPHOR+EUCAL<br />
YPTUS+MENTHOL<br />
+OTHERS Multiple<br />
EUCALYPTUS+MEN<br />
THOL+METHYL<br />
SALICYLATE+OTH<br />
ERS Multiple<br />
CAMPHOR+EUCAL<br />
YPTUS<br />
OIL+MENTHOL+ME<br />
THYLSALICYLATE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Ointment<br />
Topical<br />
Inhalational<br />
Powder<br />
Ointment<br />
Topical<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
SCIENTIFIC<br />
OFFICE (NIG)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUT<br />
ICALS LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
NOVARTIS<br />
FARMACEUTICA S.A<br />
(SPAIN)<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTICALS<br />
LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)PZ<br />
CUSSONS GH. LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 4/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/1/2013<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 7/1/2013<br />
SUPRA PHARMA<br />
LIMITED 11/1/2012<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 3/1/2013<br />
PZ CUSSONS<br />
GH. LTD 10/1/2012<br />
210<br />
MARCH 1, 2011
1800<br />
ROBB WELL BEING<br />
JUNIOR<br />
CAMPHOR+EUCAL<br />
YPTUS<br />
OIL+MENTHOL+ME<br />
THYLSALICYLATE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ointment<br />
Topical<br />
1801 ROBERTS GLYCERINE GLYCERIN 100%w/w Oral Solution<br />
1802 ROBERTS MEDICATED IRGASAN BP 300 0.5%w/w Solid Soap<br />
1803 ROCEPHIN CEFTRIAXONE 1 g<br />
1804 ROCEPHIN CEFTRIAXONE 250 mg<br />
1805 ROCEPHIN CEFTRIAXONE 500 mg<br />
1806 ROCETIL CEFUROXIME 750 mg<br />
1807 ROCETIL 1.5 CEFUROXIME 1.5 g<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
F. HOFFMAN LA-<br />
ROCHE PHARMA<br />
(SCHWEIZ) AG<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
PZ CUSSONS<br />
GH. LTD 10/1/2012<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 8/18/2013<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 4/1/2011<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
211<br />
MARCH 1, 2011
1808 ROGOPHEN<br />
1809 ROMEX COUGH<br />
1810<br />
ROMEX JUNIOR<br />
COUGH<br />
1811 RONAMLO<br />
CHLORAMPHENIC<br />
OL 0.5 %w/v Eye drops<br />
AMMONIUM<br />
CHLORIDE+MENTH<br />
OL+TOLU+SQUILL<br />
TINCTURE Multiple Syrup<br />
GUAIPHENESIN+M<br />
ENTHOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg + 0.55<br />
mg/5 mls Syrup<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
1812 RONAXICAM PIROXICAM 20 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1813 RONCET CETIRIZINE 10 mg Tablet Oral<br />
1814 RONFENAC DICLOFENAC 100 mg Suppository<br />
1815 RONFENAC DICLOFENAC 75 mg/3 ml<br />
Injectable<br />
Solution<br />
1816 RONFENAC DICLOFENAC 100 mg Tablet Oral<br />
1817 RONFENAC GEL<br />
DICLOFENAC+MEN<br />
THOL+METHYL<br />
SALICYLATE<br />
1.16 % w/w<br />
+2.00% w/w<br />
+8.0% w/w Gel Topical<br />
ROYAL<br />
MULTIPURPO<br />
SE GROUP<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
PLIVA PAKISTAN<br />
(PRIVATE) LTD.<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
BAADER SCHULZ<br />
LABORATORIES<br />
ROKMER<br />
PHARMA<br />
LIMITED 6/25/2013<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 12/31/2012<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 4/14/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 10/1/2013<br />
ROXIN GHANA<br />
LTD 10/1/2013<br />
212<br />
MARCH 1, 2011
1818 RONMAX<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg+ 400<br />
mg+ 500 mg Tablet Oral<br />
1819 RONOLOL-50 ATENOLOL 50 mg Tablet Oral<br />
1820 RONZOLE 20 OMEPRAZOLE 20 mg<br />
1821 ROSEMARY POMADE SULPHUR Multiple<br />
1822 ROVITE<br />
1823 ROX-CLAV<br />
1824 ROX-CLAV 228.5<br />
1825 ROX-CLAV 600<br />
1826 ROX-CLAV 625<br />
1827 ROX-CLAV-375<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Ointment<br />
Topical<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple Syrup<br />
AMOXICILLIN+CLA<br />
VULANIC ACID 1.2 G<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
AMOXICILLIN+CLA<br />
VULANIC ACID<br />
200 mg+ 28.5<br />
mg<br />
500 mg + 100<br />
mg<br />
Injectable<br />
Powder<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
500 mg + 125<br />
mg Tablet Oral<br />
250 mg+125<br />
mg Tablet Oral<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
BAADER SCHULZ<br />
LABORATORIES<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
ROXIN GHANA<br />
LTD 10/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
MO LOKKO<br />
ENT MO LOKKO ENT MO LOKKO ENT 10/1/2011<br />
UNI-MED<br />
INDIA UNI-MED INDIA<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
VICTORY<br />
PHARMACY 7/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED 12/31/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
213<br />
MARCH 1, 2011
1828 ROXPIK<br />
1829 ROYAMAX<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg+200<br />
mg+ 325 mg Tablet Oral<br />
200 mg/40<br />
mg/5 mls<br />
Oral<br />
Suspension<br />
1830 RUFENAC DICLOFENAC 50 mg Tablet Oral<br />
1831 SABUMOL SALBUTAMOL 4 mg Tablet Oral<br />
1832 SALBUTALIN SALBUTAMOL 2 mg/ 5 ml Syrup<br />
1833 SALBUTAMOL SALBUTAMOL 5 mg/5 mls Nebuliser<br />
1834 SALBUTAMOL SALBUTAMOL 2.5 mg/5 mls Nebuliser<br />
1835 SALBUTAMOL SALBUTAMOL<br />
100<br />
mcg/dose<br />
Inhalational<br />
Powder<br />
1836 SALBUTAMOL SALBUTAMOL 5 mg/5 mls Nebuliser<br />
1837 SALBUTAMOL SALBUTAMOL 5 mg/5 mls Nebuliser<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
ROYAL<br />
MULTIPURPO<br />
SE GROUP<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
PLIVA PAKISTAN<br />
(PRIVATE) LTD.<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
ROXIN GHANA<br />
LTD 10/1/2011<br />
ROKMER<br />
PHARMA<br />
LIMITED 2/11/2014<br />
SOCOMEX<br />
PHARMACY LTD. 9/1/2011<br />
CASSEL<br />
RESEARCH CASSEL RESEARCH<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD PVT. LTD 8/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST CHEMISTS<br />
LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
214<br />
MARCH 1, 2011
1838 SALBUTAMOL SALBUTAMOL 2.5 mg/5 mls Nebuliser<br />
1839 SALBUTAMOL SALBUTAMOL 5 mg/5 mls Nebuliser<br />
1840 SALBUTAMOL SALBUTAMOL 4 mg Tablet Oral<br />
1841 SALBUTIS INHALER SALBUTAMOL<br />
100<br />
mcg/dose<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Inhalational<br />
solution<br />
1842 SALICYCLIC ACID SALICYLIC ACID 3.5% Solid Soap<br />
1843 SALICYLIC ACID SALICYLIC ACID 2.0%w/w<br />
1844 SAMALIN COUGH Multiple Lozenges<br />
1845 SAMVIT TONIC MULTIVITAMIN Multiple Syrup<br />
1846<br />
SANATOGEN A-Z<br />
ADULT TABLETS Multiple Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
RENIE<br />
CHEMISTS<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
SHANDONG JEWIN<br />
PHARMACEUTICAL<br />
CO.<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
RENIE CHEMISTS<br />
LIMITED 2/1/2013<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S(UK) LABORATORIES(UK) PARKERSTEIN<br />
LIMITED LIMITED<br />
(GH) LTD 3/1/2012<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 11/1/2012<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 8/1/2013<br />
BONS<br />
PHARMACY LTD 5/1/2013<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
215<br />
MARCH 1, 2011
1847<br />
SANATOGEN A-Z<br />
CHILD TABLETS Multiple Tablet Oral<br />
1848 SANITRAM TRAMADOL 100 mg<br />
1849 SASTID<br />
1850 SAVLON<br />
1851 SCHERIPROCT<br />
1852 SCHERIPROCT<br />
SALICYLIC<br />
ACID+SULPHUR<br />
CETRIMIDE+CHLO<br />
RHEXIDINE<br />
CINCHOCAINE+PR<br />
EDNISOLONE<br />
CINCHOCAINE+PR<br />
EDNISOLONE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
3%w/w +<br />
5%w/w Solid Soap<br />
1.5%w/v/1.5<br />
%w/v<br />
Cream<br />
Topical<br />
1.30 mg/1<br />
mg Suppository<br />
1.9 mg/1<br />
mg/g<br />
Ointment<br />
Topical<br />
1853 SEBIVO TELBIVUDINE 20 mg/ ml Oral Solution<br />
1854 SECI SECNIDAZOLE 500 mg Tablet Oral<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
SOCOMED PHARMA<br />
PVT LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
HOCHIEZ<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2011<br />
STIEFEL<br />
LABORATORIE STIEFEL<br />
S(UK) LABORATORIES(UK) PARKERSTEIN<br />
LIMITED LIMITED<br />
(GH) LTD 7/1/2012<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
(GH. OFFICE)<br />
BELCO<br />
PHARMA PVT<br />
LTD<br />
BAYER - SCHERING<br />
PHARMA<br />
BAYER - SCHERING<br />
PHARMA<br />
NOVARTIS PHARMA<br />
STEIN AG<br />
BELCO PHARMA<br />
PVT LTD<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 6/1/2011<br />
HILLS<br />
PHARMACY 4/1/2012<br />
216<br />
MARCH 1, 2011
1855 SECNILYM SECNIDAZOLE 1 g Tablet Oral<br />
1856 SEDIMOL<br />
DICLOFENAC+LINS<br />
EED<br />
OIL+MENTHOL+ME 1%/3%/5%/10<br />
THYL SALICYLATE %w/w Gel Topical<br />
1857 SEDOZ MIDAZOLAM 1 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
1858 SELSUN Multiple Shampoo<br />
1859 SENSINIL LIDOCAINE 21.3 mg/ml<br />
1860 SEPDINE<br />
1861 SEPTRIN<br />
POVIDONE IODINE<br />
OINTMENT 5.0%<br />
w/w 5.0% w/w<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
200 mg/40<br />
mg/5 mls<br />
Injectable<br />
Suspension<br />
Ointment<br />
Topical<br />
Oral<br />
Suspension<br />
1862 SEQUIMIN METFORMIN 500 mg Tablet Oral<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
SENES<br />
PHARMA CO.<br />
LTD<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
MADRAS<br />
PHARMACEUTICALS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 7/1/2011<br />
LINCOLN<br />
PHARMACEUTICALS SENES PHARMA<br />
CO. LTD 4/1/2012<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
CLARIS<br />
LIFESCIENCES<br />
LIMITED<br />
LARK LARK<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
GLAXOWELCOME<br />
EGYPT, S.A.E.<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
DOVE<br />
PHARMACY<br />
LIMITED 10/1/2012<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
SUPRA PHARMA<br />
LIMITED 7/1/2013<br />
BIG MARON<br />
PHARMACY 5/1/2013<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/20/2015<br />
217<br />
MARCH 1, 2011
1863 SEQUIZOL ALBENDAZOLE 400 mg<br />
1864 SERETIDE DISKUS<br />
1865 SERETIDE DISKUS<br />
1866 SEROQUEL<br />
1867 SEROQUEL<br />
1868 SEROQUEL<br />
1869 SEROQUEL<br />
SALMETEROL /<br />
FLUTICASONE<br />
SALMETEROL /<br />
FLUTICASONE<br />
50 mcg/100<br />
mcg<br />
50 mcg/250<br />
mcg<br />
QUETIAPINE<br />
FUMARATE 100 mg<br />
QUETIAPINE<br />
FUMARATE 300 mg<br />
QUETIAPINE<br />
FUMARATE 25 mg<br />
QUETIAPINE<br />
FUMARATE 200 mg<br />
1870 SERTRALINE SERTRALINE 50 mg<br />
1871 SERTRALINE SERTRALINE 100 mg<br />
1872<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Chewable<br />
Inhalational<br />
Powder<br />
Inhalational<br />
Powder<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
SEVEN SEAS COD<br />
LIVER OIL COD LIVER OIL Multiple Oral Solution<br />
SEQUEL<br />
PHARMACHE<br />
M PVT LTD.<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
GLOBELA PHARMA<br />
PVT. LTD<br />
SEQUEL<br />
PHARMACHEM<br />
GHANA. LTD 4/20/2015<br />
GLAXOSMITHKLINE<br />
UK LIMITED GOKALS LTD 11/1/2011<br />
GLAXOSMITHKLINE<br />
UK LIMITED GOKALS LTD 11/1/2011<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2013<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 10/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
218<br />
MARCH 1, 2011
1873 SHALCIP CIPROFLOXACIN 500 mg Tablet Oral<br />
1874<br />
1875<br />
SHALPHATRIM<br />
PEADIATRIC<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
Oral<br />
IM 240 mg/5 mls Suspension<br />
SHALVIT<br />
MULTIVITAMIN MULTIVITAMIN Multiple Syrup<br />
1876 SHARP REJUVA<br />
1877 SHARPVITE<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN Multiple<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
1878 SHAXIN CEFTRIAXONE 1 g<br />
1879 SHINE VITAMIN C<br />
ASCORBIC ACID +<br />
CALCIUM<br />
PANTOTHENATE<br />
1880 SILVER BIRD EUCALYPTUS OIL BP<br />
1881<br />
SILVER<br />
SULPHADIAZINE<br />
500 mg + 10<br />
mg<br />
SILVER<br />
SULPHADIAZINE 1%w/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Softgel)<br />
Capsule oral<br />
(Softgel)<br />
Injectable<br />
Powder<br />
Tablet<br />
Chewable<br />
Topical<br />
solution<br />
Cream<br />
Topical<br />
SHALINA<br />
LABORATORIE<br />
S PVT. LTD.<br />
FREDUN<br />
PHARMACEUTICALS<br />
LTD<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
SHALINA<br />
LABORATORIES<br />
SHALINA PVT.<br />
LABORATORIE LTD.COPALDAS<br />
S PVT. LTD. VISRAM CO. LTD.<br />
SHARP<br />
PHARMACEUT<br />
ICALS LTD<br />
SHARP<br />
PHARMACEUT<br />
ICALS LTD<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
SHALINA SHIJIAZHUANG<br />
LABORATORIE PHARMACEUTICAL<br />
S PVT. LTD. GP CO. LTD<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
BELLS &<br />
SONS & CO<br />
LTD<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
BELLS & SONS &<br />
CO LTD<br />
SOCOMEX<br />
PHARMACY LTD. 11/1/2012<br />
SOCOMEX<br />
PHARMACY LTD. 10/6/2011<br />
SOCOMEX<br />
PHARMACY LTD. 10/1/2012<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
SOCOMEX<br />
PHARMACY LTD. 2/18/2015<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
219<br />
MARCH 1, 2011
1882 SILVERZINE<br />
SILVER<br />
SULPHADIAZINE 1%W/W<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
1883 SIMSTAR-10 SIMVASTATIN 10 mg Tablet Oral<br />
1884 SIMSTAR-20 SIMVASTATIN 20 mg Tablet Oral<br />
1885 SIMSTAR-40 SIMVASTATIN 40 mg Tablet Oral<br />
1886 SIMVA-DENK 20 SIMVASTATIN 20 mg<br />
1887 SIMVASTATIN SIMVASTATIN 20 mg<br />
1888 SINEMET 110<br />
1889 SINEMET PLUS<br />
CARBIDOPA+LEVO<br />
DOPA<br />
CARBIDOPA+LEVO<br />
DOPA<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
10 mg/100<br />
mg Tablet Oral<br />
25 mg/100<br />
mg Tablet Oral<br />
1890 SINOFLOX CIPROFLOXACIN 500 mg Tablet Oral<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
G. D. COOPER &<br />
CO. LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 9/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
GOKALS-<br />
LABOREX LTD 10/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
OSON'S CHEMIST<br />
LTD 2/1/2013<br />
220<br />
MARCH 1, 2011
1891 SINOFLOX CIPROFLOXACIN 2% USP w/v<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
1892 SINOPRIL LISINOPRIL 10 mg Tablet Oral<br />
1893 SIRDALUD TIZANIDINE 4 mg Tablet Oral<br />
1894 SIRDALUD TIZANIDINE 2 mg Tablet Oral<br />
1895 SKILAX DROPS<br />
1896 SKINEAL<br />
1897 SLOW K<br />
1898<br />
SODIUM<br />
PICOSULPHATE 7.50 mg/ml Oral Drops<br />
CLOBETASOL+KET<br />
OCONAZOLE+NEO<br />
MYCIN<br />
1%w/w/0.02<br />
5%w/w/0.75<br />
%w/w<br />
Cream<br />
Topical<br />
POTASSIUM<br />
CHLORIDE 600 mg Tablet Oral<br />
SNAKE VENOM ANTISNAKE<br />
ANTISERUM (AFRICAN) SERUM Multiple<br />
Injectable<br />
Suspension<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
GLOBAL NAPI<br />
PHARMACEUT<br />
ICALS<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
(GH. OFFICE)<br />
NOVARTIS<br />
PHARMA<br />
SERVICES<br />
(GH. OFFICE)<br />
ALMIRALL<br />
PRODESFARM<br />
A, S.L.<br />
KUNMING<br />
PHARMACEUT<br />
ICAL CORP.<br />
NOVARTIS<br />
PHARMA AG<br />
VINS<br />
BIOPRODUCT<br />
S LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
OSON'S CHEMIST<br />
LTD 7/1/2013<br />
GLOBAL NAPI<br />
PHARMACEUTICALS REHA MEDICAL<br />
SUPPLY 10/1/2012<br />
NOVARTIS PHARMA<br />
SERVICES<br />
SCIENTIFIC OFFICE<br />
(NIG)<br />
NOVARTIS PHARMA<br />
SERVICES<br />
SCIENTIFIC OFFICE<br />
(NIG)<br />
ALMIRALL<br />
PRODESFARMA,<br />
S.L.<br />
KUNMING<br />
PHARMACEUTICAL<br />
CORP.<br />
CIBA<br />
LABORATORIES<br />
VINS BIOPRODUCTS<br />
LTD<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 3/1/2013<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 3/1/2013<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
THE PILL BOX<br />
LTD. 7/1/2011<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
CHI MEDICAL<br />
(GH) LTD 7/1/2011<br />
221<br />
MARCH 1, 2011
1899 SOCOGYL PLUS<br />
1900 SOCOMOL<br />
1901 SODIUM CHLORIDE<br />
1902 SODIUM CHLORIDE<br />
1903 SODIUM CHLORIDE<br />
1904<br />
SODIUM CHLORIDE &<br />
GLUCOSE<br />
1905 SOFKOF<br />
FURAZOLIDONE+M<br />
ETRONIDAZOLE<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
30 mg+150<br />
mg<br />
30 mg+ 200<br />
mg+ 325 mg<br />
SODIUM<br />
CHLORIDE 0.9%w/v<br />
SODIUM<br />
CHLORIDE 0.9% w/v<br />
SODIUM<br />
CHLORIDE 0.9%w/v<br />
GLUCOSE+SODIUM<br />
CHLORIDE<br />
CHLORPHENIRAMI<br />
NE+DEXTROMETH<br />
ORPHAN+PHENYL<br />
EPHRINE<br />
0.9% & 5%<br />
w/v<br />
1906 SOLCER OMEPRAZOLE 40 mg<br />
2 mg + 10 mg<br />
+ 5 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
Large<br />
Volume<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Softgel)<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
FRESENIUS<br />
KABI INDIA<br />
PVT. LIMITED<br />
FRESNIUS<br />
MAFATLAL<br />
MEDICALS<br />
LTD.<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
FRESENIUS<br />
KABI INDIA<br />
PVT. LIMITED<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
SOCOMED PHARMA<br />
PVT LTD<br />
SOCOMED PHARMA<br />
PVT LTD<br />
FRESENIUS KABI<br />
INDIA PVT LTD<br />
FRESNIUS<br />
MAFATLAL<br />
MEDICALS LTD.<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
FRESENIUS KABI<br />
INDIA PVT.<br />
LIMITED<br />
XL LABORATORIES<br />
PVT LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
BEDITA<br />
PHARMACY 6/1/2013<br />
BEDITA<br />
PHARMACY 7/1/2013<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 4/1/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
GR PHARMA<br />
LTD. 12/1/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 1/7/2013<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 7/1/2013<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 11/1/2013<br />
222<br />
MARCH 1, 2011
1907 SOLCOSERYL<br />
1908<br />
SOLCOSERYL DENTAL<br />
ADH PASTE<br />
1909 SOLCOSERYL JELLY<br />
1910 SOLUBLE ASPIRIN<br />
1911 SOLUBLE ASPIRIN<br />
1912 SOLUBLE ASPIRIN<br />
1913 SOLUBLE ASPIRIN<br />
1914 SOLU-MEDROL<br />
1915 SONA<br />
DEPROTEINIZED<br />
CALF BLOOD 20% w/w<br />
DEPROTEINIZED<br />
SANGUIN<br />
EXTRACT+POLIDO<br />
CANOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ointment<br />
Topical<br />
5%<br />
w/v/1%w/v Paste<br />
DEPROTEINIZED<br />
CALF BLOOD 20% w/w Gel Topical<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
ACETYLSALICYLIC<br />
ACID 75 mg<br />
Tablet<br />
Dispersible<br />
Tablet<br />
Dispersible<br />
Tablet<br />
Dispersible<br />
ACETYLSALICYLIC<br />
ACID 75 mg Tablet Oral<br />
METHYLPREDNISO<br />
LONE 40 mg/ ml<br />
HYDROCORTISONE<br />
+NEOMYCIN<br />
Injectable<br />
Solution<br />
15 mg + 3.5<br />
mg ENT Solution<br />
SOLCO BASLE<br />
LTD SOLCO BASLE LTD<br />
SOLCO BASLE<br />
LTD SOLCO BASLE LTD<br />
SOLCO BASLE<br />
LTD SOLCO BASLE LTD<br />
BEDITA<br />
PHARMACY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
PFIZER WEST<br />
AFRICA<br />
(NIGERIA)<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
PFIZER<br />
MANUFACTURING<br />
BELGIUM NV<br />
EGYPTIAN<br />
INTERNATION EGYPTIAN<br />
AL<br />
INTERNATIONAL<br />
PHARMACEUT PHARMACEUTICAL<br />
ICAL IND. CO. IND. CO.<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 3/1/3009<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
223<br />
MARCH 1, 2011
1916 SPANIL<br />
1917 SPASMINTAS<br />
HYOSCINE<br />
BUTYLBROMIDE 10 mg<br />
HYOSCINE<br />
BUTYLBROMIDE 10 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
1918 SPIRONOLACTONE SPIRONOLACTONE 25 mg Tablet Oral<br />
1919 SPIRONOLACTONE SPIRONOLACTONE 50 mg Tablet Oral<br />
1920 SPIRONOLACTONE SPIRONOLACTONE 100 mg Tablet Oral<br />
1921 SPIRONOLACTONE SPIRONOLACTONE 25 mg Tablet Oral<br />
1922 SPIRONOLACTONE SPIRONOLACTONE 100 mg Tablet Oral<br />
1923 SPIRONOLACTONE SPIRONOLACTONE 50 mg Tablet Oral<br />
1924 SPIRONOLACTONE SPIRONOLACTONE 25 mg Tablet Oral<br />
1925 SPIRONOLACTONE SPIRONOLACTONE 50 mg Tablet Oral<br />
BEXIMCO<br />
PHARMACEUT<br />
ICALS<br />
LIMITED<br />
INTAS<br />
PHARMACEUT<br />
ICALS LTD-<br />
MATODA<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
BEXIMCO<br />
PHARMACEUTICALS<br />
LIMITED<br />
INTAS<br />
PHARMACEUTICALS<br />
LTD -DEHRADUN<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
BEXIMCO<br />
PHARMACEUTIC<br />
ALS LIMITED 9/1/2011<br />
GR PHARMA<br />
LTD. 7/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
224<br />
MARCH 1, 2011
1926 SPIRONOLACTONE SPIRONOLACTONE 100 mg Tablet Oral<br />
1927 SPIRONOLACTONE SPIRONOLACTONE 50 mg Tablet Oral<br />
1928 SPIZEF CEFUROXIME 750 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
1929 SPIZEF CEFUROXIME 250 mg Tablet Oral<br />
1930 SPIZEF CEFUROXIME 500 mg Tablet Oral<br />
1931<br />
SPLENDO<br />
DISINFECTANT (MALE<br />
& FEMALE) IODINE<br />
10 mg/100<br />
ml<br />
1932 SPORANOX ITRACONAZOLE 100 mg<br />
1933 SPRUKCEE VITAMIN C 500 mg<br />
Topical<br />
Spray<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet<br />
Chewable<br />
1934 SPRUKFEN IBUPROFEN 400 mg Tablet Oral<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ORCHID<br />
HEALTHCARE<br />
ORCHID<br />
HEALTHCARE<br />
ORCHID<br />
HEALTHCARE<br />
CHUZHOU<br />
IRIS FAMILY<br />
PRODUCTS<br />
CO. LTD.<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD.<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ORCHID<br />
HEALTHCARE<br />
ORCHID<br />
HEALTHCARE<br />
ORCHID<br />
HEALTHCARE<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 6/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 4/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 4/1/2013<br />
CHUZHOU IRIS<br />
FAMILY PRODUCTS<br />
CO. LTD. IONA PHARMACY 1/17/2015<br />
JANSSEN-CILAG<br />
S.p.A.(ITALY)<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 7/1/2011<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
225<br />
MARCH 1, 2011
1935<br />
1936<br />
1937<br />
1938<br />
SPRUKFIELD<br />
AMOXICILLIN AMOXICILLIN 250 mg<br />
SPRUKFIELD<br />
AMOXICILLIN AMOXICILLIN 500 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
SPRUKFIELD<br />
CIPROFLOXACIN CIPROFLOXACIN 500 mg Tablet Oral<br />
SPRUKFIELD CO-<br />
TRIMOXAZOLE<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
IM<br />
400 mg/80<br />
mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1939 STAGYL SECNIDAZOLE 1000 mg Tablet Oral<br />
1940 STARCOLD<br />
1941<br />
1942<br />
CHLORPHENIRAMI<br />
NE+PARACETAMO<br />
L+PSEUDOEPHEDR<br />
INE<br />
2 mg/500<br />
mg/30 mg Tablet Oral<br />
STARWIN<br />
METRONIDAZOLE METRONIDAZOLE 200 mg Tablet Oral<br />
STARWIN MILK OF<br />
MAGNESIA<br />
MAGNESIUM<br />
HYDROXIDE 8.56%w/v Mixtures<br />
1943 STAVUDINE STAVUDINE 15 mg<br />
1944 STAVUDINE STAVUDINE 20 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
GB PHARMA<br />
(GH) LTD<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
SPRUKFIELD (UK)<br />
SARL (TOGO)<br />
SPRUKFIELD (UK)<br />
SARL (TOGO)<br />
SPRUKFIELD (UK)<br />
SARL (TOGO)<br />
SPRUKFIELD (UK)<br />
SARL (TOGO)<br />
ESKAY<br />
THERAPEUTICS 8/1/2011<br />
ESKAY<br />
THERAPEUTICS 8/1/2011<br />
ESKAY<br />
THERAPEUTICS 8/1/2011<br />
ESKAY<br />
THERAPEUTICS 8/1/2011<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 7/1/2011<br />
STARWIN<br />
PRODUCTS LTD.<br />
STARWIN<br />
PRODUCTS LTD.<br />
STARWIN<br />
PRODUCTS LTD.<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2012<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2011<br />
STARWIN<br />
PRODUCTS LTD. 9/1/2011<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 5/1/2011<br />
226<br />
MARCH 1, 2011
1945 STAVUDINE STAVUDINE 30 mg<br />
1946 STAVUDINE STAVUDINE 40 mg<br />
1947<br />
1948<br />
STAVUDINE+LAMIVUDI<br />
NE<br />
STAVUDINE+LAMIVUDI<br />
NE<br />
1949 STEFERON<br />
1950 STEP<br />
1951<br />
STERILISED WATER<br />
FOR INJECTION<br />
1952 STERILIZED WATER<br />
LAMIVUDINE+STA<br />
VUDINE<br />
LAMIVUDINE+STA<br />
VUDINE<br />
FOLIC<br />
ACID+IRON(III)<br />
HYDROXIDE<br />
POLYMALTOSE<br />
COMPLEX<br />
CAMPHOR+EUCAL<br />
YPTUS<br />
OIL+MENTHOL+ME<br />
THYLSALICYLATE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
30 mg/150<br />
mg Tablet Oral<br />
40 mg/150<br />
mg Tablet Oral<br />
0.5 mg+ 50<br />
mg /5 ml Syrup<br />
WATER FOR<br />
INJECTION 100 % v/v<br />
WATER FOR<br />
INJECTION N/A<br />
Inhalational<br />
Vapour<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
GLOSTEVE<br />
(PH) LTD<br />
OSON'S<br />
CHEMIST LTD<br />
SVIZERA<br />
EUROPE BV<br />
MARCK<br />
BIOSCIENCE<br />
(PARENTERAL<br />
S) INDIA LTD<br />
STRIDES ARCOLAB<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
STRIDES ARCOLAB<br />
LIMITED<br />
GLOSTEVE (PH)<br />
LTD<br />
BURAPHA<br />
DISPENSARY CO<br />
LTD<br />
NIRMA<br />
HEALTHCARE<br />
LIMITED<br />
MARCK BIOSCIENCE<br />
(PARENTERALS)<br />
INDIA LTD<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 4/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 11/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 12/31/2011<br />
GLOSTEVE (PH)<br />
LTD 7/1/2013<br />
OSON'S CHEMIST<br />
LTD 11/1/2013<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 2/1/2012<br />
GR PHARMA<br />
LTD. 11/1/2011<br />
1953 STESOLID DIAZEPAM 5 mg Tablet Oral DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2012<br />
1954 STESOLID DIAZEPAM 10 mg Tablet Oral DANNEX LTD DANNEX LTD DANNEX LTD 10/1/2012<br />
227<br />
MARCH 1, 2011
1955 STOMACAINE<br />
1956 STREPTOL<br />
ALUMINIUM<br />
HYDROXIDE<br />
GEL+MAGNESIUM<br />
HYDROXIDE+OXET<br />
HAZAINE Multiple<br />
1957 STREPTOMYCIN STREPTOMYCIN 1g<br />
1958 STREPTOMYCIN STREPTOMYCIN 1g<br />
1959<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
EUCALYPTUS+MEN<br />
THOL Multiple Lozenges<br />
STREPTOMYCIN<br />
INJECTION STREPTOMYCIN 1 g<br />
1960 STRICORT 100 HYDROCORTISONE 100 mg/vial<br />
1961 STRIXONE CEFTRIAXONE 1 g<br />
1962 STRIZEF CEFUROXIME 750 mg<br />
1963 STROBIN<br />
EUCALYPTUS+MEN<br />
THOL<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
(lyophilized)<br />
0.3%<br />
w/w/0.4<br />
%w/w Lozenges<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
MILAN<br />
LABOTRATOR<br />
IES (INDIA)<br />
SVIZERA<br />
EUROPE BV<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
STRIDES<br />
ARCOLAB<br />
LIMITED<br />
TOBINCO<br />
PHARMACY<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
MILAN<br />
LABOTRATORIES<br />
(INDIA)<br />
SVIZERA LABS PVT.<br />
LTD<br />
TROGE MEDICAL<br />
GMBH<br />
UNICHEM<br />
GHANA LTD. 6/1/2011<br />
OSON'S CHEMIST<br />
LTD 8/1/2011<br />
NATIONAL<br />
TUBERCULOSIS<br />
PROGRAMME 5/1/2011<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
ERNEST CHEMISTS<br />
LIMITED 11/22/2011<br />
STRIDES ARCOLAB<br />
LIMITED<br />
QUANTUM LIFE<br />
SCIENCES PVT LTD<br />
(STRIDES<br />
ARCOLAB)<br />
STRIDES ARCOLAB<br />
LIMITED<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 4/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 6/1/2012<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2013<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
228<br />
MARCH 1, 2011
1964 STROBIN INHALER<br />
CAMPHOR+EUCAL<br />
YPTUS<br />
OIL+MENTHOL+ME<br />
THYLSALICYLATE Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Inhalational<br />
Vapour<br />
1965 STUGERON CINNARIZINE 25 mg Tablet Oral<br />
1966 SUBSYDE-CR DICLOFENAC 100 mg<br />
1967 SUCCINYLCHOLINE<br />
1968 SUCRAFIL O GEL<br />
SUXAMETHONIUM<br />
CHLORIDE<br />
500 mg/ 10<br />
ml<br />
OXETACAINE+SUC<br />
RAFATE 20 mg/1 g<br />
1969 SUDOCREM Multiple<br />
1970 SULBACIN<br />
1971 SULBACIN<br />
1972 SULDOX<br />
AMPICILLIN+SULB<br />
ACTAM 1g/0.5 g<br />
AMPICILLIN+SULB<br />
ACTAM 0.5/0.25 g<br />
PYRIMETHAMINE+<br />
SULPHADOXINE<br />
Capsule<br />
Delayed<br />
Release<br />
Injectable<br />
Solution<br />
Oral<br />
Suspension<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
RAPTAKOS<br />
BRETT & CO.<br />
LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
JANSSEN-CILAG<br />
FARMACEUTICA, ABBA<br />
LDA<br />
SCIENTIFIC<br />
QUELZE(PORTUGAL PROMOTION<br />
)<br />
LTD. 7/1/2011<br />
RAPTAKOS BRETT<br />
& CO. LIMITED<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES<br />
AKRO -<br />
PHARMACEUT<br />
ICALS LTD<br />
(UK)<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
HARMONY<br />
CHEMISTS LTD 3/1/2012<br />
4/1/2012<br />
GR PHARMA<br />
LTD. 8/1/2011<br />
KORLYNS<br />
PHARMACEUTIC<br />
ALS INT. LTD. 11/1/2011<br />
GR PHARMA<br />
LTD. 2/1/2012<br />
GR PHARMA<br />
LTD. 9/1/2013<br />
500 mg/25<br />
mg Tablet Oral DANNEX LTD DANNEX LTD DANNEX LTD 9/1/2012<br />
229<br />
MARCH 1, 2011
1973 SULPHATHIAZOLE SULPHATHIAZOLE 500 mg Tablet Oral<br />
1974 SULPHATHIAZOLE SULPHATHIAZOLE 500 mg Tablet Oral<br />
1975 SUMO ACTIVE<br />
1976 SUPADEX<br />
AMINO<br />
ACIDS+CYPROHEP<br />
TADINE+VITAMINS Multiple Syrup<br />
AMINO<br />
ACIDS+LIVER<br />
EXTRACT+MINERA<br />
LS+VITAMINS Multiple Syrup<br />
1977 SUPAVITA MULTIVITAMIN Multiple Syrup<br />
1978 SUPER APETI<br />
1979<br />
1980<br />
1981<br />
1982<br />
SUPER ATLAS<br />
LAXATIVE<br />
SUPER ATLAS<br />
MULTIVITAMIN<br />
SUPER ATLAS<br />
MULTIVITAMIN &<br />
MINERALS<br />
CYPROHEPTADINE<br />
+MULTIVITAMINS Multiple Tablet Oral<br />
HYDROANTHRACE<br />
NE GLYCOSIDES 15 mg Tablet Oral<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
SUPER ATLAS PURE<br />
COD LIVER OIL COD LIVER OIL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
3500 iu/400<br />
iu/5 mls Oral Solution<br />
ESKAY<br />
THERAPEUTIC ESKAY<br />
S<br />
THERAPEUTICS<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
GB PHARMA<br />
(GH) LTD<br />
TRADE WINDS<br />
CHEMIST LTD<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
TRADE WINDS<br />
CHEMIST LTD<br />
GEO MEDICORE<br />
LIMITED<br />
ESKAY<br />
THERAPEUTICS 9/1/2013<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
GB PHARMA<br />
(GH) LTD 7/1/2011<br />
TRADE WINDS<br />
CHEMIST LTD 10/1/2012<br />
GEO MEDICORE<br />
LIMITED 8/1/2012<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 12/31/2012<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)PZ<br />
CUSSONS GH. LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
230<br />
MARCH 1, 2011
1983<br />
SUPER ATLAS PURE<br />
COD LIVER OIL COD LIVER OIL<br />
1984 SUPRAPEN<br />
AMOXYCILLIN+FLU<br />
CLOXACILLIN<br />
0.32<br />
mls/capsule<br />
250 mg/250<br />
mg<br />
1985 SUPRAVITE MULTIVITAMIN Multiple Syrup<br />
1986 SUPRIN<br />
ACETYLSALICYLIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL<br />
1987 SURFAZ VAGINAL CLOTRIMAZOLE 100 mg<br />
1988 SURFAZ-SN<br />
1989<br />
SUXAMETHONIUM<br />
CHLORIDE<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+GENTAMYCIN Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
250 mg/125<br />
mg/25 mg Tablet Oral<br />
SUXAMETHONIUM<br />
CHLORIDE 100 mg/ 2ml<br />
Tablet<br />
Vaginal<br />
Ointment<br />
Topical<br />
Inhalational<br />
solution<br />
PZ CUSSONS<br />
GH. LTD<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
PHARMANOV<br />
A LTD.<br />
FRANCO-<br />
INDIAN<br />
PHARMACEUT<br />
ICALS PVT.<br />
LTD<br />
FRANCO-<br />
INDIAN<br />
PHARMACEUT<br />
ICALS PVT.<br />
LTD<br />
PZ CUSSONS IND.<br />
LTD (NIGERIA)PZ<br />
CUSSONS GH. LTD<br />
MEDREICH<br />
LIMITED<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
FRANCO-INDIAN<br />
PHARMACEUTICALS<br />
PVT. LTD<br />
FRANCO-INDIAN<br />
PHARMACEUTICALS<br />
PVT. LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD NYCOMED<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
REGENT<br />
PHARMACY 7/1/2011<br />
REGENT<br />
PHARMACY 4/1/2013<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
231<br />
MARCH 1, 2011
1990<br />
1991<br />
SUXAMETHONIUM<br />
CHLORIDE-FRESENIUS<br />
SYMBICORT<br />
TURBUHALER<br />
SUXAMETHONIUM<br />
CHLORIDE 100 mg/ 2ml<br />
BUDESONIDE+FOR<br />
MOTEROL<br />
160/4.5<br />
mcg/dose<br />
1992 SYNTOCINON OXYTOCIN 5 iu/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Inhalational<br />
solution<br />
Inhalational<br />
Powder<br />
Injectable<br />
Solution<br />
1993 TACIZOL ALBENDAZOLE 400 mg Tablet Oral<br />
1994 TACIZOL ALBENDAZOLE 100 mg/5 ml<br />
1995 TADOL TRAMADOL 50 mg<br />
Oral<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
1996 TAGERA FORTE SECNIDAZOLE 1g Tablet Oral<br />
1997 TAMOXIFEN TAMOXIFEN 20 mg Tablet Oral<br />
1998 TAMOXIFEN TAMOXIFEN 20 mg Tablet Oral<br />
BODENE (Pty)<br />
LTD,<br />
TRADING AS<br />
INTRAMED<br />
ASTRAZENEC<br />
A UK LTD<br />
NOVARTIS<br />
PHARMA<br />
GmbH<br />
GB PHARMA<br />
(GH) LTD<br />
GB PHARMA<br />
(GH) LTD<br />
KRKA d.d.<br />
NOVO<br />
MESTO,<br />
SLOVENIA<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
ASTRAZENECA UK<br />
LTD<br />
NOVARTIS FARMA<br />
SPA (ITALY)<br />
HILLS<br />
PHARMACY 11/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 1/13/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 2/1/2012<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 2/1/2013<br />
MISSION VIVACARE<br />
LIMITED<br />
KRKA d.d. NOVO<br />
MESTO, SLOVENIA<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
GB PHARMA<br />
(GH) LTD 2/1/2013<br />
ATLAS<br />
PHARMACY<br />
LIMITED 3/1/2012<br />
GR PHARMA<br />
LTD. 11/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
232<br />
MARCH 1, 2011
1999 TAMOXIFEN TAMOXIFEN 20 mg Tablet Oral<br />
2000 TAMOXIFEN TAMOXIFEN 20 mg Tablet Oral<br />
2001 TAMOXIFEN TAMOXIFEN 10 mg Tablet Oral<br />
2002 TAMSULOSIN TAMSULOSIN 400 mcg Tablet Oral<br />
2003 TANZOL ALBENDAZOLE<br />
400 mg/10<br />
mls<br />
2004 TASTYMOL PARACETAMOL 250 mg/5 mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
2005 TAVANIC LEVOFLOXACIN 500 mg Tablet Oral<br />
2006 TAVANIC LEVOFLOXACIN 500 mg<br />
Injectable<br />
Solution<br />
2007 TAVEGYL CLEMASTINE 1 mg Tablet Oral<br />
ROCK<br />
CHEMISTS<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
BEDITA<br />
PHARMACY<br />
G. D. COOPER &<br />
CO. LIMITED<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
BETA<br />
HEALTHCARE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
SOCOMEX<br />
PHARMACY LTD. 4/1/2013<br />
KINAPHARMA<br />
LTD 10/1/2011<br />
AVENTIS PHARMA<br />
DEUTSCHLAND GOKALS LTD 8/1/2013<br />
AVENTIS PHARMA<br />
DEUTSCHLAND GOKALS LTD 8/1/2013<br />
NOVARTIS PHARMA<br />
GmbH<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 2/1/2012<br />
233<br />
MARCH 1, 2011
2008 TAXOTERE DOCETAXOL 20 mg<br />
2009 TAXOTERE DOCETAXOL 80 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
2010 T-BRAN TOBRAMYCIN 0.3% w/v Eye drops<br />
2011 TCP LIQUID<br />
HALOGENATED<br />
PHENOLS+PHENOL<br />
+SODIUM<br />
SALICYLATE Multiple Oral Solution<br />
2012 TCP MEDICATED SALICYLIC ACID 0.254 %w/w Solid Soap<br />
2013 TEARS NATURALE II<br />
DEXTRAN+HYDRO<br />
XYPROPYL<br />
METHYL<br />
CELLULOSE 0.1%w/v Eye drops<br />
2014 TEBIF 125 TERBINAFINE 125 mg Tablet Oral<br />
2015 TEBIF 250 TERBINAFINE 250 mg Tablet Oral<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
NEIMETH<br />
INT'L PHARM.<br />
PLC<br />
AVENTIS PHARMA,<br />
DAGENHAM<br />
AVENTIS PHARMA,<br />
DAGENHAM<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
NEIMETH INT'L<br />
PHARM. PLC<br />
GOKALS-<br />
LABOREX LTD 6/1/2011<br />
GOKALS-<br />
LABOREX LTD 6/1/2011<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 2/1/2012<br />
ELLIOT<br />
IRVING<br />
LIMITED SIGMA SOAP LTD. PNT PHARMACY 11/1/2011<br />
ALCON-<br />
COUVREUR<br />
ALCON-<br />
COUVREUR<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
GR PHARMA<br />
LTD. 11/1/2013<br />
GR PHARMA<br />
LTD. 11/1/2013<br />
234<br />
MARCH 1, 2011
2016 TEEDAR<br />
DIPHENHYDRAMIN<br />
E+PARACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
120 mg/12.5<br />
mg/5 ml Syrup<br />
2017 TEGRETOL CARBAMAZEPINE 2 %w/v Syrup<br />
2018 TEGRETOL CARBAMAZEPINE 200 mg Tablet Oral<br />
2019 TEGRETOL CR CARBAMAZEPINE 200 mg<br />
2020 TEGRETOL CR CARBAMAZEPINE 400 mg<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Extended<br />
Realese<br />
2021 TELFAST FEXOFENADINE 180 mg Tablet Oral<br />
2022 TELFAST FEXOFENADINE 120 mg Tablet Oral<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 3/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
AVENTIS<br />
AVENTIS PHARMA<br />
DEUTSCHLANDAVE<br />
NTIS<br />
PHARMA(USA)MAR<br />
ION MERRELL<br />
PHARMA(USA) BOUGAOIN S.A. GOKALS LTD 9/1/2011<br />
AVENTIS<br />
AVENTIS PHARMA<br />
DEUTSCHLANDAVE<br />
NTIS<br />
PHARMA(USA)MAR<br />
ION MERRELL<br />
PHARMA(USA) BOUGAOIN S.A. GOKALS LTD 8/1/2013<br />
235<br />
MARCH 1, 2011
2023 TEMODAL TEMOZOLAMIDE 5 mg<br />
2024 TEMODAL TEMOZOLAMIDE 20 mg<br />
2025 TEMODAL TEMOZOLAMIDE 100 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
UNIVERSITY OF<br />
IOWA (USA)ORION<br />
CORPORATION<br />
(FINLAND)SCHERI<br />
NG-PLOUGH LABO<br />
N.V.(BELGIUM)SG<br />
S LAB SIMON SA GOKALS LTD 7/1/2012<br />
UNIVERSITY OF<br />
IOWA (USA)ORION<br />
CORPORATION<br />
(FINLAND)SCHERI<br />
NG-PLOUGH LABO<br />
N.V.(BELGIUM)SG<br />
S LAB SIMON SA GOKALS LTD 7/1/2012<br />
UNIVERSITY OF<br />
IOWA (USA)ORION<br />
CORPORATION<br />
(FINLAND)SCHERI<br />
NG-PLOUGH LABO<br />
N.V.(BELGIUM)SG<br />
S LAB SIMON SA GOKALS LTD 7/1/2012<br />
236<br />
MARCH 1, 2011
2026 TEMODAL TEMOZOLAMIDE 250 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2027 TENLIN MEBENDAZOLE 500 mg Tablet Oral<br />
2028<br />
2029<br />
2030<br />
2031<br />
TENOFOVIR<br />
DISOPROXIL<br />
FUMARATE TENOFOVIR 300 mg<br />
TENOFOVIR&EMTRICIT<br />
ABINE<br />
TENOFOVIR&LAMIVUD<br />
INE<br />
TENOFOVIR+EFAVIREN<br />
Z+EMTRICITABINE<br />
2032 TENORET<br />
2033 TENORETIC<br />
EMTRICITABINE+T<br />
ENOFOVIR<br />
LAMIVUDINE+TEN<br />
OFOVIR<br />
EFAVIRENZ+EMTRI<br />
TABINE+TENOFOV<br />
IR<br />
ATENOLOL+CHLO<br />
RTHALIDONE<br />
ATENOLOL+CHLO<br />
RTHALIDONE<br />
200 mg/300<br />
mg<br />
300 mg/300<br />
mg<br />
600 mg+ 200<br />
mg +300 mg<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
50 mg/12.5<br />
mg Tablet Oral<br />
100 mg/25<br />
mg Tablet Oral<br />
2034 TENORMIN ATENOLOL 50 mg Tablet Oral<br />
SCHERING-<br />
PLOUGH<br />
(PTY) LTD<br />
(SOUTH<br />
AFRICA)<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
UNIVERSITY OF<br />
IOWA (USA)ORION<br />
CORPORATION<br />
(FINLAND)SCHERI<br />
NG-PLOUGH LABO<br />
N.V.(BELGIUM)SG<br />
S LAB SIMON SA GOKALS LTD 7/1/2012<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
RENIE CHEMISTS<br />
LIMITED 10/1/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 6/19/2013<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 4/1/2011<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
237<br />
MARCH 1, 2011
2035 TENORMIN ATENOLOL 100 mg Tablet Oral<br />
2036 TENSI FORTE<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
ASTRAZENEC<br />
A UK LTD<br />
LISINOPRIL +<br />
AMLODIPINE 5 mg/5 mg Tablet Oral KOJACH LTD<br />
2037 TERAZOSIN TERAZOSIN 2 mg Tablet Oral<br />
2038 TERAZOSIN TERAZOSIN 2 mg Tablet Oral<br />
2039 TERBINAFINE TERBINAFINE 250 mg Tablet Oral<br />
2040 TERBINAFINE TERBINAFINE 250 mg Tablet Oral<br />
2041 TESPRIL LISINOPRIL 10 mg Tablet Oral<br />
2042 TETGLOB<br />
2043 TETRA<br />
2044 TETRACYCLINE<br />
TETANUS<br />
IMMUNOGLOBULI<br />
N 250 iu/vial<br />
TETRACYCLINE<br />
HYDROCHLORIDE 250 mg<br />
TETRACYCLINE<br />
HYDROCHLORIDE 250 mg<br />
Injectable<br />
Suspension<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
HILLS<br />
PHARMACY<br />
BHARAT<br />
SERUMS AND<br />
VACCINES<br />
LTD.<br />
SPRUKFIELD<br />
(UK) SARL<br />
(TOGO)<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
MARION BIOTECH<br />
PVT. LTD. KOJACH LTD 4/1/2013<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
BELCO PHARMA<br />
PVT LTD<br />
BHARAT SERUMS<br />
AND VACCINES<br />
LTD.<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
HILLS<br />
PHARMACY 7/1/2012<br />
FAR EAST<br />
MERCANTILE 6/1/2013<br />
SPRUKFIELD (UK)<br />
SARL (TOGO) 12/31/2011<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 2/1/2013<br />
238<br />
MARCH 1, 2011
2045 TETRAKIN<br />
2046 TG-TOR 10<br />
2047 TG-TOR-20<br />
2048<br />
2049<br />
2050<br />
TETRACYCLINE<br />
HYDROCHLORIDE 250 mg<br />
ATORVASTATIN<br />
CALCIUM 10mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
ATORVASTATIN<br />
CALCIUM 20 mg Tablet Oral<br />
THIOPENTONE<br />
SODIUM THIOPENTAL 1 g/20 ml<br />
THIOPENTONE<br />
SODIUM THIOPENTAL 1 g<br />
THIOPENTONE<br />
SODIUM THIOPENTAL 0.5 g<br />
2051 TIDACT CLINDAMYCIN 150 mg<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2052 TIMOLOL TIMOLOL 0.5%w/v Eye drops<br />
2053 TIZIGESIC<br />
TIZANIDINE<br />
HYDROCHLORIDE 4mg Tablet Oral<br />
2054 TIZIGESIC TIZANIDINE 2 mg Tablet Oral<br />
2055<br />
TOBIN'S BAYCARE<br />
GRIPE WATER<br />
ANISE+BICARBONA<br />
TE+DILL+MENTHO<br />
L Multiple Oral Solution<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
KINAPHARMA<br />
LTD 8/1/2012<br />
GR PHARMA<br />
LTD. 4/1/2011<br />
GR PHARMA<br />
LTD. 4/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA ROCK CHEMISTS 2/1/2012<br />
Y. S. P.<br />
INDUSTRIES<br />
(M) SDN. BHD<br />
BEDITA<br />
PHARMACY<br />
Y. S. P.<br />
INDUSTRIES (M)<br />
SDN. BHD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
TOBINCO<br />
PHARMACY<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
RENIE CHEMISTS<br />
LIMITED 2/1/2013<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
GR PHARMA<br />
LTD. 9/1/2013<br />
GR PHARMA<br />
LTD. 9/1/2013<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
239<br />
MARCH 1, 2011
2056<br />
2057<br />
TOBIN'S COD LIVER<br />
OIL COD LIVER OIL 300 mg<br />
TOBRADEX<br />
OPTHALMIC<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
DEXAMETHASONE<br />
+TOBRAMYCIN 3 mg/1 mg Eye drops<br />
2058 TOBREX TOBRAMYCIN 0.3 %w/w Eye ointment<br />
2059 TOBREX TOBRAMYCIN 0.3 %w/w Eye drops<br />
2060 TOBRIN TOBRAMYCIN 3.0 mg/ ml Eye drops<br />
2061 TOBVITAL MULTIVITAMIN Multiple Tablet Oral<br />
TOBINCO<br />
PHARMACY<br />
ALCON-<br />
COUVREUR<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
GUJARAT LIQUI<br />
PHARMACAPS (P)<br />
LTD.<br />
ALCON-<br />
COUVREUR<br />
ALCON<br />
PHARMACEUTICALS<br />
ALCON<br />
PHARMACEUTICALS<br />
EGYPTIAN<br />
INTERNATION EGYPTIAN<br />
AL<br />
INTERNATIONAL<br />
PHARMACEUT PHARMACEUTICAL<br />
ICAL IND. CO. IND. CO.<br />
TOBINCO<br />
PHARMACY<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 9/1/2012<br />
RENIE CHEMISTS<br />
LIMITED 11/1/2012<br />
TOBINCO<br />
PHARMACY 10/1/2012<br />
240<br />
MARCH 1, 2011
2062 TODAY PREMIUM NONOXYNOL-9 5% w/w<br />
2063 TOGAMYCIN SPECTINOMYCIN 2 g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Tablet<br />
Vaginal<br />
Injectable<br />
Powder<br />
2064 TOLBUTAMIDE TOLBUTAMIDE 500 mg Tablet Oral<br />
2065 TOLBUTAMIDE TOLBUTAMIDE 500 mg Tablet Oral<br />
2066 TOLBUTAMIDE TOLBUTAMIDE 500 mg Tablet Oral<br />
2067 TOLBUTAMIDE TOLBUTAMIDE 500 mg Tablet Oral<br />
2068 TOOCHE TOOTHACHE<br />
MENTHOIL+CLOVE<br />
+TOLU BALSAM<br />
+CAJAPUT OIL Multiple Oral Solution<br />
2069 TOROCEF-1 CEFTRIAXONE 1000 mg<br />
Injectable<br />
Solution<br />
BLISS<br />
CHEMICALS &<br />
PHARMACEUT<br />
ICALS INDIA<br />
LTD<br />
PFIZER<br />
REGIONAL<br />
OFFICE<br />
(Ghana)<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
BLISS CHEMICALS &<br />
PHARMACEUTICALS<br />
INDIA LTD<br />
PFIZER<br />
MANUFACTURING<br />
BELGIUM NV<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
HEALTHILIFE<br />
PHARMA PVT.<br />
LTD(INDIA)<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 2/1/2012<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
UNICHEM<br />
GHANA LTD. 12/1/2012<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 12/31/2012<br />
241<br />
MARCH 1, 2011
2070 TOT'HEMA<br />
2071 TOZZAR-H<br />
COPPER + IRON +<br />
MANGANESE<br />
HYDROCHLOROTH<br />
IAZIDE+LOSARTAN<br />
2072 TRACAM PIROXICAM 20mg<br />
2073 TRACONATIL-S ITRACONAZOLE 100 mg<br />
2074 TRADICLO DICLOFENAC 50 mg<br />
2075 TRAMADOL TRAMADOL 50 mg<br />
2076 TRAMADOL TRAMADOL 50 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
0.7 mg + 50<br />
mg + 1.33 mg Oral Solution<br />
50.00 mg +<br />
12.50 mg Tablet Oral<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet<br />
Enteric<br />
Coated<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2077 TRANEXAMIC ACID TRANEXAMIC ACID 500 mg Tablet Oral<br />
LABORATOIRE<br />
INNOTECH<br />
INTERNATION<br />
AL<br />
TORRENT<br />
PHARMACEUT<br />
ICALS LTD.<br />
TRADE WINDS<br />
CHEMIST LTD<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY<br />
(GH) LTD<br />
PRASHI<br />
PHARMA PVT<br />
LTD<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
INNOTHERA<br />
CHOUZY<br />
(INNOTHERA<br />
GROUP)<br />
GOKALS-<br />
LABOREX LTD 9/1/2013<br />
TORRENT<br />
PHARMACEUTICALS<br />
LTD. GOKALS LTD 11/1/2012<br />
TRADE WINDS<br />
CHEMIST LTD<br />
LAGRAY CHEMICAL<br />
COMPANY (GH)<br />
LTD<br />
PRASHI PHARMA<br />
PVT LTDSAPHIRE<br />
LIFESCIENCES PVT.<br />
LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD CAMPDALE<br />
TRADE WINDS<br />
CHEMIST LTD 10/1/2011<br />
LAGRAY<br />
CHEMICAL<br />
COMPANY (GH)<br />
LTD 3/1/2013<br />
TRADE WINDS<br />
CHEMIST LTD 4/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
242<br />
MARCH 1, 2011
2078 TRANEXAMIC ACID TRANEXAMIC ACID 500 mg Tablet Oral<br />
2079 TRAVATAN TRAVOPROST 0.004% Eye drops<br />
2080 TRAVOCORT<br />
2081 TRAVOGEN<br />
2082 TRES-ORIX FORTE<br />
2083 TRIACTIN<br />
DIFLUCORTOLONE<br />
+ISOCONAZOLE 10 mg/1 mg<br />
ISOCONAZOLE<br />
NITRATE 10 mg/g<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
Cream<br />
Topical<br />
CYPROHEPTADINE<br />
+MULTIVITAMIN+P<br />
ROTEIN Multiple Syrup<br />
CAFFEINE+EPHED<br />
RINE<br />
HCL+PARACETAM<br />
OL<br />
500 mg/10<br />
mg/ 30 mg Tablet Oral<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
ALCON<br />
PHARMACEUT<br />
ICALS<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
ALMIRALL<br />
PRODESFARM<br />
A, S.L.<br />
ATLANTIC<br />
PHARMACEUT<br />
ICALS<br />
LABORS.<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
ALCON-<br />
COUVREUR<br />
BAYER - SCHERING<br />
PHARMA<br />
BAYER - SCHERING<br />
PHARMA<br />
ALMIRALL<br />
PRODESFARMA,<br />
S.L.<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
PARACELSUS<br />
PHARMACY<br />
&MARKETING<br />
COMPANY<br />
LIMITED 12/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 9/1/2012<br />
PHARMANOVA<br />
LTD. 9/1/2012<br />
243<br />
MARCH 1, 2011
2084 TRIAPIN<br />
2085 TRIAPIN MITE<br />
FELODIPINE+RAMI<br />
PRIL 5 mg/5 mg Tablet Oral<br />
FELODIPINE+RAMI<br />
PRIL<br />
2086 TRIAXONE CEFTRIAXONE 1000 mg<br />
2087 TRIDERM<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
2.5 mg/2.5<br />
mg Tablet Oral<br />
BETAMETHASONE<br />
+CLIOQUINOL+GE 0.61 mg/10<br />
NTAMYCIN+TOLNA mg/1 mg/10<br />
FTATE<br />
mg<br />
Injectable<br />
Powder<br />
Cream<br />
Topical<br />
2088 TRIFLUOPERAZINE TRIFLUOPERAZINE 5 mg Tablet Oral<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
AVENTIS<br />
PHARMA<br />
(PTY)<br />
LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
SENES<br />
PHARMA CO.<br />
LTD<br />
ERNEST<br />
CHEMISTS<br />
LIMITED<br />
ASTRAZENECA UK<br />
LTDASTRAZENECA<br />
AB(Sweden)CHINO<br />
IN<br />
PHARMACEUTICALS<br />
& CHEMICAL<br />
WORKS PRIVATE<br />
CO. LTDCHINOIN<br />
PHARMACEUTICALS<br />
& CHEMICAL<br />
WORKS PRIVATE<br />
CO. LTD GOKALS LTD 8/1/2013<br />
ASTRAZENECA UK<br />
LTDASTRAZENECA<br />
AB(Sweden)AVEN<br />
TIS PHARMA (PTY)<br />
LIMITEDAVENTIS<br />
PHARMA<br />
DEUTSCHLAND GOKALS LTD 8/1/2013<br />
AUROBINDO<br />
PHARMA LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 7/1/2012<br />
LINCOLN<br />
PHARMACEUTICALS SENES PHARMA<br />
CO. LTD 6/1/2012<br />
ERNEST CHEMISTS<br />
LIMITED<br />
ERNEST<br />
CHEMISTS<br />
LIMITED 8/1/2011<br />
244<br />
MARCH 1, 2011
2089 TRIHEPTA<br />
2090 TRIODA<br />
2091 TRIOMUNE 30<br />
AMINO<br />
ACIDS+CYPROHEP<br />
TADINE+VITAMINS Multiple Syrup<br />
BECLOMETHASON<br />
E+CLOTRIMAZOLE<br />
+GENTAMYCIN<br />
1%w/w/0.1%<br />
w/w/0.05%w<br />
/w<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Cream<br />
Topical<br />
LAMIVUDINE+NEVI<br />
RAPINE+STAVUDIN 30 mg/150<br />
E<br />
mg/200 mg Tablet Oral<br />
2092 TRITACE RAMIPRIL 2.5 mg Tablet Oral<br />
2093 TRITACE RAMIPRIL 5 mg Tablet Oral<br />
2094 TRITACE RAMIPRIL 10 mg Tablet Oral<br />
2095 TRO-PADOL-FLU<br />
ASCORBIC<br />
ACID+CAFFEINE+P<br />
ARACETAMOL<br />
350 mg/2<br />
mg/100 mg Tablet Oral<br />
TRADE WINDS<br />
CHEMIST LTD<br />
ALLY PHARMA<br />
OPTIONS PVT<br />
LTD<br />
TRADE WINDS<br />
CHEMIST LTD<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
CIPLA<br />
LIMITED CIPLA LIMITED<br />
TRADE WINDS<br />
CHEMIST LTD 10/1/2012<br />
DAAMASS CO.<br />
LIMITED 4/1/2012<br />
HEALTHCARE<br />
SERVICES LTD 9/1/2012<br />
AVENTIS AVENTIS PHARMA<br />
PHARMA(USA) DEUTSCHLAND GOKALS LTD 8/1/2013<br />
AVENTIS AVENTIS PHARMA<br />
PHARMA(USA) DEUTSCHLAND GOKALS LTD 9/1/2013<br />
SANOFI<br />
AVENTIS<br />
NIGERIA<br />
LIMITED<br />
SANOFI-AVENTIS<br />
S.p.A<br />
TROGE<br />
MEDICAL<br />
GMBH MEDOPHARM<br />
GOKALS-<br />
LABOREX LTD 12/1/2013<br />
ANSDAN CEDAR<br />
CO. LTD 6/1/2011<br />
245<br />
MARCH 1, 2011
2096 TRUVADA<br />
2097<br />
TRYPSIN-<br />
CHYMOTRYPSIN<br />
2098 TURA GERMICIDAL<br />
EMTRICITABINE+T<br />
ENOFOVIR<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
200 mg/300<br />
mg Tablet Oral<br />
CHYMOTRYPSIN+T<br />
RYPSIN 5000 I.U Tablet Oral<br />
ALLANTOIN+TRICL<br />
OSAN+VITAMIN E multiple Solid Soap<br />
2099 TYFLOX-500 CIPROFLOXACIN 500 mg Tablet Oral<br />
2100 TYLENOL PARACETAMOL 350 mg Suppository<br />
2101 ULTRAVIST 300 IOPROMIDE 0.623 mg/ml<br />
2102 UMOXAZOLE<br />
Injectable<br />
Solution<br />
SULFAMETHOXAZ<br />
OLE+TRIMETHOPR<br />
Oral<br />
IM 240 mg/5 mls Suspension<br />
ASPEN<br />
PHARMACARE<br />
HOLDINGS<br />
LTD.<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
TURA<br />
INTERNATION<br />
AL LTD<br />
AJANTA<br />
PHARMA<br />
(MAURITIUS)<br />
LTD.<br />
JANSSEN-<br />
CILAG<br />
S.p.A.(ITALY)<br />
BAYER -<br />
SCHERING<br />
PHARMA<br />
PATHEON INC.<br />
(Canada)ALTANA<br />
PHARMA<br />
ORANIENBURG<br />
GmbHPHARMACAR<br />
E LIMITEDASPEN<br />
PHARMACARE<br />
HOLDINGS LTD.<br />
GOLDSHIELD<br />
PHARMACEUTICALS<br />
LTD<br />
TURA<br />
INTERNATIONAL<br />
LTD<br />
AJANTA PHARMA<br />
(MAURITIUS) LTD.<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
BAYER - SCHERING<br />
PHARMA<br />
UMEDICA<br />
LABORATORIE AXON <strong>DRUG</strong>S<br />
S PVT. LTD. PRIVVATE LTD.<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/1/2012<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
FOREWIN GH<br />
LTD 7/1/2011<br />
NEO PHARMA<br />
CENTRE LIMITED 7/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 8/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 4/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 10/1/2013<br />
246<br />
MARCH 1, 2011
2103 UMOXIL AMOXICILLIN 125 mg/5ml<br />
2104 UNIENZYME cMPS<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
ACTIVATED<br />
CHARCOAL+FUNG<br />
AL<br />
DIASTASE+PAPAIN<br />
+SIMETHICONE Multiple Tablet Oral<br />
2105 UPROGESIC NAPROXEN 500 mg Tablet Oral<br />
2106 UROCTAL NORFLOXACIN 400 mg Tablet Oral<br />
2107 UROGRAFIN<br />
MEGLUMINE<br />
AMIDOTRIZOATE+<br />
SODIUM<br />
AMIDOTRIZOATE 76%w/v<br />
Injectable<br />
Suspension<br />
2108 UTIRIZINE CETIRIZINE 10 mg Tablet Oral<br />
2109 VAGINAX-100 CLOTRIMAZOLE 100 mg<br />
2110 VAGIRON CLOTRIMAZOLE 100 mg<br />
Tablet<br />
Vaginal<br />
Tablet<br />
Vaginal<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
UNICHEM UNICHEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
ALMIRALL<br />
PRODESFARM<br />
A, S.L.<br />
ALMIRALL<br />
PRODESFARMA,<br />
S.L.<br />
BAYER -<br />
SCHERING BAYER - SCHERING<br />
Ghana office. PHARMA<br />
UMEDICA UMEDICA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
RONAK EXIM<br />
PRIVATE<br />
LIMITED<br />
SALOM<br />
PHARMACY<br />
LIMITED 11/1/2013<br />
GR PHARMA<br />
LTD. 8/1/2013<br />
SALOM<br />
PHARMACY<br />
LIMITED 10/1/2011<br />
KAMA<br />
INDUSTRIES<br />
LTD. 5/1/2012<br />
BAYER -<br />
SCHERING<br />
PHARMA AFRICA<br />
GMBH 4/1/2011<br />
SALOM<br />
PHARMACY<br />
LIMITED 9/1/2012<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 2/1/2013<br />
RONAK EXIM<br />
PRIVATE LIMITED<br />
ROXIN GHANA<br />
LTD 12/31/2011<br />
247<br />
MARCH 1, 2011
2111 VENTOLIN SALBUTAMOL 2mg/5 mls Syrup<br />
2112 VENTOLIN EVOHALER SALBUTAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
100<br />
mcg/actuatio Inhalational<br />
n<br />
solution<br />
2113 VENTOLINE SALBUTAMOL 5 mg/5 mls Nebuliser<br />
2114 VENTOLINE SALBUTAMOL 2.5 mg/5 mls Nebuliser<br />
2115 VERAFLOX CIPROFLOXACIN 0.3%<br />
Ear/Eye<br />
Drops<br />
2116 VERAPAMIL VERAPAMIL 40 mg Tablet Oral<br />
2117 VERAPAMIL VERAPAMIL 80 mg Tablet Oral<br />
2118 VERMOX MEBENDAZOLE 20 mg/ml<br />
Oral<br />
Suspension<br />
2119 VERMOX MEBENDAZOLE 500 mg Tablet Oral<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
ROCK<br />
CHEMISTS<br />
ROCK<br />
CHEMISTS<br />
INDUSKEN<br />
PHARMACEUT<br />
ICALS PVT<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
JANSSEN<br />
PHARMACEUT<br />
ICA N. V.<br />
GLAXOWELCOME<br />
EGYPT, S.A.E.<br />
GLAXOWELLCOME<br />
PRODUCTION<br />
(FRANCE)<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
CIRON <strong>DRUG</strong>S &<br />
PHARMACEUTICALS<br />
PVT LTD<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
JANSSEN<br />
PHARMACEUTICA<br />
N. V.<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 10/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 10/1/2013<br />
ABBA<br />
SCIENTIFIC<br />
PROMOTION<br />
LTD. 10/1/2013<br />
248<br />
MARCH 1, 2011
2120 VIANDINE Z<br />
2121<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS MULTIPLE Syrup<br />
VICKS MEDICATED<br />
ASCORBIC<br />
ACID+LEMON<br />
(BLUE & LEMON PLUS) OIL+MENTHOL Multiple Lozenges<br />
2122 VIFEX<br />
2123 VIKONON TONIC<br />
BROMHEXINE+GU<br />
AIFENESIN+SALBU<br />
TAMOL<br />
MULTIVITAMIN+MI<br />
NERALS Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
1 mg/2 mg/<br />
50 mg/5ml Syrup<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2124 VIN-C ASCORBIC ACID 100 mg Tablet Oral<br />
2125<br />
2126<br />
VINCRISTINE<br />
SULPHATE VINCRISTINE 2 mg/ml<br />
VINCRISTINE<br />
SULPHATE VINCRISTINE 1 mg/ml<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
2127 VIRACEPT NELFINAVIR 250 mg Tablet Oral<br />
NETAB<br />
PHARMACY<br />
PROCTER &<br />
GAMBLE<br />
INTERNATION<br />
AL<br />
OPERATIONS<br />
SA<br />
EMCURE<br />
PHARMACEUT<br />
ICALS<br />
WALLACE<br />
PRODUCTS<br />
UK LTD<br />
GR<br />
INDUSTRIES<br />
LTD.<br />
MERCURY<br />
ANTIBIOTICS PVT.<br />
LTD.<br />
PROCTER &<br />
GAMBLE NIGERIA<br />
LIMITED<br />
NETAB<br />
PHARMACY 7/1/2011<br />
TRANS GLOBAL<br />
SHIPPING LTD 2/13/2012<br />
EMCURE<br />
PHARMACEUTICALS PHARMANOVA<br />
LTD. 6/1/2013<br />
SAVOY<br />
LABORATORIES(INT<br />
) LTD<br />
GR INDUSTRIES<br />
LTD.<br />
SERUM<br />
INSTITUTE OF SERUM INSTITUTE<br />
INDIA OF INDIA<br />
ROCK<br />
CHEMISTS<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PHARMA<br />
(SCHWEIZ)<br />
AG<br />
UNICHEM<br />
GHANA LTD. 8/1/2012<br />
GR INDUSTRIES<br />
LTD. 7/1/2011<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2011<br />
DEVATS INDIA PVT<br />
LTD ROCK CHEMISTS 2/1/2012<br />
F. HOFFMAN LA-<br />
ROCHE<br />
DIAGNOSTICS<br />
GmbH<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 10/1/2013<br />
249<br />
MARCH 1, 2011
2128 VIREAD TENOFOVIR 300 mg Tablet Oral<br />
2129 VIROL BLOOD TONIC<br />
LIVER<br />
EXTRACT+VITAMI<br />
NS+MINERALS Multiple Syrup<br />
2130 VITACAN CLOTRIMAZOLE 1% w/w<br />
2131 VITAFORCE<br />
2132 VITAL X<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Ointment<br />
Topical<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Syrup<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple Syrup<br />
2133 VITALEX MULTIVITAMIN 100 mg Oral Drops<br />
2134 VITALMINE MULTIVITAMIN Multiple Syrup<br />
ASPEN<br />
PHARMACARE<br />
HOLDINGS<br />
LTD.<br />
AYRTON<br />
<strong>DRUG</strong> MFG.<br />
COMPANY<br />
LTD<br />
BETAMED<br />
INTERNATION<br />
AL FZE<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
TOBINCO<br />
PHARMACY<br />
LONDON<br />
UNITED<br />
EXPORTS<br />
LIMITED<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
PATHEON INC.<br />
(Canada)ALTANA<br />
PHARMA<br />
ORANIENBURG<br />
GmbHPHARMACAR<br />
E LIMITEDASPEN<br />
PHARMACARE<br />
HOLDINGS LTD.<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
SOCOMED PHARMA<br />
PVT LTD<br />
AGLOWMED<br />
LIMITED<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
ZHONGNUO<br />
PHARMACEUTICALS<br />
(SHIJAIAZHUANG)<br />
CO. LTD<br />
GEO MEDICORE<br />
LIMITED<br />
PHYTO-RIKER<br />
(GIHOC)<br />
PHARMACEUTIC<br />
ALS LTD 12/1/2012<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD 3/1/2012<br />
HOCHIEZ<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2013<br />
UNICHEM<br />
GHANA LTD. 10/1/2012<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
UNICHEM<br />
GHANA LTD. 11/1/2012<br />
GEO MEDICORE<br />
LIMITED 3/18/2014<br />
250<br />
MARCH 1, 2011
2135 VITAMIN B COMPLEX<br />
2136 VITAMIN B COMPLEX<br />
2137 VITAMIN B COMPLEX<br />
VITAMIN B<br />
COMPLEX Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
VITAMIN B<br />
COMPLEX Multiple Tablet Oral<br />
VITAMIN B<br />
COMPLEX Multiple Tablet Oral<br />
2138 VITAMIN B1 VITAMIN B1 10 mg Tablet Oral<br />
2139 VITAMIN B-COMPLEX<br />
VITAMIN B<br />
COMPLEX Multiple Tablet Oral<br />
2140 VITAMIN K1 VITAMIN K1 10 mg/ mls<br />
2141 VITAMIN K1 VITAMIN K1 10 mg/ mls<br />
2142 VITAMIN K1 VITAMIN K1 10 mg/ mls<br />
2143 VITAMIN K1 VITAMIN K1 1 mg/ mls<br />
2144 VITAMIN K1 VITAMIN K1 10 mg/ mls<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
TROGE<br />
MEDICAL<br />
GMBH<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
PHARMANOV<br />
A LTD.<br />
SISHUI XIERKANG<br />
PHARMACEUTICAL<br />
COMPANY<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
ATLANTIC<br />
PHARMACEUTICALS<br />
LABORS.<br />
DAAMASS CO.<br />
LIMITED 7/1/2011<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
PHARMANOVA<br />
LTD. 6/1/2012<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS ROCK CHEMISTS 3/1/2012<br />
LETAP<br />
PHARMACEUT<br />
ICALS LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
LETAP<br />
PHARMACEUTICALS<br />
LTD<br />
SHREE GANESH<br />
PHARMACEUTICALS<br />
ROCK<br />
CHEMISTS STEROP OVERSEAS<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA<br />
ROCK<br />
CHEMISTS AMSTEL PHARMA<br />
LETAP<br />
PHARMACEUTIC<br />
ALS LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 2/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 2/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 2/1/2012<br />
251<br />
MARCH 1, 2011
2145 VITANOL<br />
MULTIVITAMIN+MI<br />
NERALS Multiple Tablet Oral<br />
2146 VIT-B-DENK MULTIVITAMIN Multiple Tablet Oral<br />
2147 VITIRON SUSCAPS<br />
2148 VL CHILDREN COUGH<br />
2149 VL NAVRAN<br />
FOLIC<br />
ACID+IRON+MULTI<br />
VITAMINS Multiple<br />
CHLORPHENIRAMI<br />
NE+DEXTROMETH<br />
ORPHAN+GUAIPH<br />
ENESIN+OTHERS Multiple Syrup<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
50 mg + 650<br />
mg Tablet Oral<br />
2150 VOLFAST DICLOFENAC 50 mg/sachet Oral Solution<br />
2151 VOLPE<br />
DICLOFENAC+PAR<br />
ACETAMOL<br />
500 mg + 50<br />
mg Tablet Oral<br />
WALTER<br />
RITTER<br />
PHARMACEUT<br />
ICA<br />
DENK<br />
PHARMA<br />
GmbH & Co.<br />
KG<br />
WALTER RITTER<br />
PHARMACEUTICA<br />
DENK PHARMA<br />
GmbH & Co. KG<br />
Capsule oral<br />
(Hard<br />
gelatin) MEPHA LTD. MEPHA LTD.<br />
NAVKETAN<br />
PHARMA PVT. NAVKETAN<br />
LTD PHARMA PVT. LTD<br />
NAVKETAN<br />
PHARMA PVT. NAVKETAN<br />
LTD PHARMA PVT. LTD<br />
MIPHARM<br />
S.P.A MIPHARM S.P.A<br />
GUANDONG<br />
MEDICINES &<br />
HEALTH<br />
PRODUCTS<br />
I/E CORP<br />
JIANGXI XIER<br />
KANGTAI<br />
PHARMACEUTICAL<br />
CO. LTD<br />
UNICHEM<br />
GHANA LTD. 4/1/2012<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 12/31/2011<br />
PHARMA INFO<br />
CONSULT 7/1/2013<br />
VICBARNS<br />
PHARMACY<br />
LIMITED 11/1/2013<br />
VICBARNS<br />
PHARMACY<br />
LIMITED 11/1/2013<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
252<br />
MARCH 1, 2011
2152 VOLRIN DICLOFENAC 75 mg/3 ml<br />
2153 VOLTAREN DICLOFENAC 50 mg<br />
2154 VOLTAREN EMUGEL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
Tablet film<br />
coated<br />
DICLOFENAC<br />
DIETHYLAMMONIU<br />
M 1% w/w Gel Topical<br />
2155 VOLTAREN RETARD DICLOFENAC 100 mg<br />
2156 VOLTAREN SR DICLOFENAC 75 mg<br />
2157 VOLUVEN<br />
POLYHYDROXYET<br />
HYL STARCH<br />
60 g/1000<br />
mls<br />
Tablet<br />
Extended<br />
Realese<br />
Tablet<br />
Extended<br />
Realese<br />
Injectable<br />
Solution<br />
2158 WARFARIN WARFARIN 3 mg Tablet Oral<br />
2159 WARFARIN WARFARIN 5 mg Tablet Oral<br />
2160 WARFARIN WARFARIN 5 mg Tablet Oral<br />
GUANDONG<br />
MEDICINES &<br />
HEALTH<br />
PRODUCTS<br />
I/E CORP<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS<br />
FARMA SPA<br />
(ITALY)<br />
FRESENIUS<br />
KABI (PTY)<br />
LTD (SOUTH<br />
AFRICA)<br />
EAST<br />
CANTONMEN<br />
TS<br />
PHARMACY<br />
BEDITA<br />
PHARMACY<br />
ROCK<br />
CHEMISTS<br />
SISHUI XIERKANG<br />
PHARMACEUTICAL<br />
COMPANY<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS PHARMA<br />
AG<br />
NOVARTIS FARMA<br />
SPA (ITALY)<br />
BODENE (Pty) LTD,<br />
TRADING AS<br />
INTRAMED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
BEDITA<br />
PHARMACY 11/1/2011<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 12/1/2012<br />
NOVARTIS<br />
PHARMA<br />
SERVICES (GH.<br />
OFFICE) 12/1/2012<br />
HILLS<br />
PHARMACY 12/31/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 11/1/2011<br />
BEDITA<br />
PHARMACY 10/3/2013<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
253<br />
MARCH 1, 2011
2161 WARFARIN WARFARIN 5 mg Tablet Oral<br />
2162 WARFARIN WARFARIN 3 mg Tablet Oral<br />
2163 WARFARIN WARFARIN 5 mg Tablet Oral<br />
2164 WARFARIN WARFARIN 3 mg Tablet Oral<br />
2165 WARFARIN WARFARIN 5 mg Tablet Oral<br />
2166 WARFARIN WARFARIN 5 mg Tablet Oral<br />
2167<br />
WATER FOR<br />
INJECTION<br />
2168 WHITFIELDS<br />
WATER FOR<br />
INJECTION 100 %v/v<br />
BENZOIC<br />
ACID+SALICYLIC<br />
ACID<br />
6%w/w,<br />
3%w/w<br />
2169 WIDACAM PIROXICAM 20 mg<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
UNICOM<br />
CHEMIST<br />
LIMITED CAMPDALE<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD<br />
JEBINA<br />
PHARMACY<br />
LTD<br />
ROYAL DACH<br />
PHARMACEUT<br />
ICAL LTD IMPRES<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
G. D. COOPER &<br />
CO. LIMITED<br />
UNICOM<br />
CHEMIST<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
LYMENS<br />
MEDICAL<br />
SUPPLIES<br />
LIMITED 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
JEBINA<br />
PHARMACY LTD 4/1/2012<br />
ROYAL DACH<br />
PHARMACEUTIC<br />
AL LTD 11/1/2011<br />
Ointment<br />
Topical DANNEX LTD DANNEX LTD DANNEX LTD 4/1/2013<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
CLARION<br />
INDIA CLARION INDIA<br />
WIDAMA<br />
PHARMACY CO.<br />
LTD 10/1/2011<br />
254<br />
MARCH 1, 2011
2170 WIDAKID TONIC<br />
2171 WIDAPAIN<br />
2172 WIN-DEPIN<br />
2173 WIN-DEPIN<br />
VITAMINS+PROTEI<br />
N+IRON+MINERAL<br />
S Multiple Syrup<br />
CAFFEINE+IBUPRO<br />
FEN+PARACETAM<br />
OL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
30 mg+400<br />
mg+500 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 10 mg Tablet Oral<br />
AMLODIPINE<br />
BESILATE 5 mg Tablet Oral<br />
2174 WINOLON NALIDIXIC ACID 500 mg Tablet Oral<br />
2175<br />
WOKADINE<br />
ANTISEPTIC GARGLE POVIDONE-IODINE 1.00% w/v Oral Solution<br />
2176 WORMAZOL MEBENDAZOLE 100 mg<br />
2177 WORMBASE ALBENDAZOLE 400 mg<br />
Tablet<br />
Chewable<br />
Tablet<br />
Chewable<br />
2178 WORMBAT-400 ALBENDAZOLE 400 mg Tablet Oral<br />
2179 WORMBEN MEBENDAZOLE 500mg Tablet Oral<br />
2180 WORMEE 4 ALBENDAZOLE 400 mg Tablet Oral<br />
MERCURY<br />
ANTIBIOTICS<br />
PVT. LTD.<br />
MERCURY<br />
ANTIBIOTICS PVT.<br />
LTD.<br />
MERCURY MERCURY<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
GB PHARMA<br />
(uk) LTD.<br />
STARWIN<br />
PRODUCTS<br />
LTD.<br />
WOCKHARDT<br />
LTD (INDIA)<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
WIDA<br />
PHARMACY CO.<br />
LTD 10/1/2011<br />
WIDA<br />
PHARMACY CO.<br />
LTD 10/1/2011<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 4/1/2012<br />
LINCOLN<br />
PHARMACEUTICALS GB PHARMA<br />
(GH) LTD 4/1/2012<br />
STARWIN<br />
PRODUCTS LTD.<br />
WOCKHARDT LTD<br />
(INDIA)<br />
STARWIN<br />
PRODUCTS LTD. 5/1/2011<br />
SHARP<br />
PHARMACEUTIC<br />
ALS LTD 10/1/2011<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 8/1/2013<br />
MERCURY MERCURY<br />
LABORATORIE LABORATORIES<br />
S LTD LTD<br />
UNICHEM<br />
INDUSTRIES<br />
LTD<br />
UNICHEM<br />
INDUSTRIES LTD<br />
KINAPHARMA<br />
LTD KINAPHARMA LTD<br />
TOBINCO<br />
PHARMACY<br />
ALLY PHARMA<br />
OPTIONS PVT LTD<br />
BASE PHARMACY<br />
LIMITED 9/1/2011<br />
UNICHEM<br />
GHANA LTD. 6/1/2013<br />
KINAPHARMA<br />
LTD 4/1/2013<br />
TOBINCO<br />
PHARMACY 10/1/2012<br />
255<br />
MARCH 1, 2011
2181<br />
WORMEE 4<br />
SUSPENSION ALBENDAZOLE 100 mg/5 ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
2182 WORMEX PIPERAZINE 750 mg Syrup<br />
2183 WORMPLEX 400 ALBENDAZOLE 100 mg/5ml<br />
2184 WORMZAP ALBENDAZOLE<br />
400 mg/20<br />
ml<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
2185 WORMZAP ALBENDAZOLE 400 mg Tablet Oral<br />
2186 X'AMCLOX ORAL<br />
AMPICILLIN+CLOX<br />
ACILLIN<br />
2187 XARELTO RIVAROXABAN 10 mg<br />
2188 XATRAL LP ALFUZOSIN 10 mg<br />
125mg/125m<br />
g/5mls<br />
2189 X'CLOX ORAL CLOXACILLIN 125mg/5mls<br />
Powder for<br />
Oral<br />
Suspension<br />
Tablet film<br />
coated<br />
Tablet film<br />
coated<br />
Powder for<br />
Oral<br />
Suspension<br />
TOBINCO<br />
PHARMACY<br />
M & G<br />
PHARMACEUT<br />
ICALS LTD<br />
MISSION<br />
VIVACARE<br />
LIMITED<br />
GR<br />
INDUSTRIES<br />
LTD.<br />
GR<br />
INDUSTRIES<br />
LTD.<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
M & G<br />
PHARMACEUTICALS<br />
LTD<br />
MISSION VIVACARE<br />
LIMITED<br />
TOBINCO<br />
PHARMACY 4/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 9/1/2012<br />
ESKAY<br />
THERAPEUTICS 10/1/2013<br />
GR INDUSTRIES<br />
LTD. 10/1/2013<br />
GR INDUSTRIES<br />
LTD.<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
BAYER -<br />
SCHERING<br />
PHARMA BAYER - SCHERING<br />
AFRICA GMBH PHARMA<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP<br />
(FRANCE)<br />
SPARSH BIO-<br />
TECH PVT<br />
LTD<br />
GR PHARMA<br />
LTD. 6/1/2013<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
BAYER -<br />
SCHERING<br />
Ghana office. 6/1/2012<br />
SANOFI-<br />
SYNTHELABO<br />
GROUP (FRANCE) GOKALS LTD 8/1/2013<br />
SPARSH BIO-TECH<br />
PVT LTD<br />
TOBINCO<br />
PHARMACY 5/1/2011<br />
256<br />
MARCH 1, 2011
2190 XELODA CAPECITABINE 500 mg Tablet Oral<br />
2191 XENICAL ORLISTAT 120 mg<br />
2192 XONE CEFTRIAXONE 250 mg<br />
2193 XONE CEFTRIAXONE 500 mg<br />
2194 XONE CEFTRIAXONE 1 g<br />
2195 X-PRES<br />
CAFFEINE+CHLOR<br />
PHENIRAMINE+PA<br />
RACETAMOL<br />
2196 XYLOCAINE LIDOCAINE 10%v/v<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
Injectable<br />
Powder<br />
30 mg+2 mg+<br />
500 mg Tablet Oral<br />
Topical<br />
Spray<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
F. HOFFMAN<br />
LA-ROCHE<br />
PRODUCTS<br />
(PTY) LTD.<br />
(SOUTH<br />
AFRICA)<br />
F. HOFFMAN LA-<br />
ROCHE S.p.A.<br />
(Italy)<br />
F. HOFFMAN LA-<br />
ROCHE PRODUCTS<br />
(PTY) LTD. (SOUTH<br />
AFRICA)<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD<br />
ROCK<br />
CHEMISTS<br />
2197 XYLO-MEPHA XYLOMETAZOLINE 0.1%w/v Nasal Spray MEPHA LTD.<br />
2198 XYLO-MEPHA XYLOMETAZOLINE 0.05%w/v Nasal Spray MEPHA LTD.<br />
XL LABORATORIES<br />
PVT. LTD.<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 10/1/2011<br />
F. HOFFMAN LA-<br />
ROCHE GHANA<br />
LTD 12/1/2012<br />
FAR EAST<br />
MERCANTILE 6/1/2011<br />
FAR EAST<br />
MERCANTILE 6/1/2011<br />
FAR EAST<br />
MERCANTILE 6/1/2011<br />
RIAK<br />
(SINGAPORE)<br />
PTE LTD 10/1/2011<br />
DEREK CLARKE<br />
PHARMACEUTICALS<br />
(UK) ROCK CHEMISTS 11/1/2011<br />
MERCK KGaA<br />
(GERMANY)<br />
MERCK KGaA<br />
(GERMANY)<br />
PHARMA INFO<br />
CONSULT 4/1/2011<br />
PHARMA INFO<br />
CONSULT 4/1/2011<br />
257<br />
MARCH 1, 2011
2199 ZADITEN KETOTIFEN 1 mg/5 mls Syrup<br />
2200 ZADITEN KETOTIFEN 1 mg Tablet Oral<br />
2201 ZAMOXYL AMOXICILLIN 500 mg<br />
2202 ZANTAC RANITIDINE 25 mg/ml<br />
2203 ZEMAN<br />
2204 ZENGLOBIN<br />
AMINO<br />
ACIDS+MINERALS+<br />
MULTIVITAMINS Multiple<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Injectable<br />
Solution<br />
Capsule oral<br />
(Softgel)<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2205 ZENTEL ALBENDAZOLE 200 mg Tablet Oral<br />
2206 ZERODOL ACECLOFENAC 100 mg Tablet Oral<br />
2207 ZESTORETIC-20<br />
HYDROCHLOROTH<br />
IAZIDE+LISINOPRI<br />
L<br />
20 mg/12.5<br />
mg Tablet Oral<br />
NOVARTIS<br />
PHARMA AG<br />
NOVARTIS PHARMA<br />
S.A.S<br />
NOVARTIS<br />
PHARMA AG FAMAR FRANCE<br />
SHALINA SHALINA<br />
LABORATORIE LABORATORIES<br />
S PVT. LTD. PVT. LTD.<br />
GLAXOSMITH<br />
KLINE LTD<br />
(ITALY)<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD<br />
JENBURKT<br />
PHARMACEUT<br />
ICALS LTD<br />
GLAXOSMITH<br />
KLINE<br />
INTERNATION<br />
AL<br />
GLAXOSMITHKLINE<br />
LTD (ITALY)<br />
MEGA LIFE<br />
SCIENCES<br />
(THAILAND)<br />
JENBURKT<br />
PHARMACEUTICALS<br />
LTD<br />
MEDREICH<br />
LIMITEDMEDREICH<br />
LIMITED<br />
IPCA LABS.<br />
LTD. IPCA LABS. LTD.<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENECA<br />
AB(Sweden)<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
MEDVIEW<br />
PHARMA<br />
SERVICES LTD. 2/1/2012<br />
SOCOMEX<br />
PHARMACY LTD. 10/1/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
MEGA<br />
LIFESCIENCES<br />
PTY LTD 8/1/2012<br />
MEYIRI<br />
PHARMACY<br />
LIMITED 9/15/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 10/1/2011<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 7/1/2013<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
258<br />
MARCH 1, 2011
2208 ZESTRIL LISINOPRIL 5 mg Tablet Oral<br />
2209 ZESTRIL LISINOPRIL 10 mg Tablet Oral<br />
2210 ZESTRIL LISINOPRIL 20 mg Tablet Oral<br />
2211 ZIDOCUM MIDAZOLAM 5 mg/ml<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Solution<br />
2212 ZIDOVIR ZIDOVUDINE 50 mg/5 mls Oral Solution<br />
2213 ZIDOVUDINE ZIDOVUDINE 100 mg<br />
2214 ZIDOVUDINE ZIDOVUDINE 300 mg<br />
2215 ZIF<br />
FOLIC<br />
ACID+IRON+ZINC<br />
500 mcg/150<br />
mg/61.80 mg<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
Tablet film<br />
coated<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
2216 ZILOCAM MELOXICAM 7.5 mg Tablet Oral<br />
2217 ZILOCAM MELOXICAM 15 mg Tablet Oral<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D.<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
ASTRAZENECA<br />
AB(Sweden)<br />
LEK<br />
PHARMACEUTICALS<br />
D. D.<br />
CIPLA<br />
LIMITED CIPLA LIMITED<br />
AUROBINDO<br />
PHARMA<br />
LIMITED<br />
AUROBINDO<br />
PHARMA LIMITED<br />
MATRIX MATRIX<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
SQUARE<br />
PHARMACEUT<br />
ICALS<br />
SQUARE<br />
PHARMACEUTICALS<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. CIPLA LIMITED<br />
LEK<br />
PHARMACEUT<br />
ICALS D. D. CIPLA LIMITED<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
ASTRAZENECA<br />
UK LTD 2/1/2012<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
HEALTHCARE<br />
SERVICES LTD 9/1/2012<br />
AUROBINDO<br />
PHARMA<br />
LTD.(Ghana) 7/1/2011<br />
VICDORIS<br />
PHARMACEUTIC<br />
ALS LIMITED 12/1/2012<br />
MEDICHEM<br />
PHARMACEUTIC<br />
ALS LTD. 6/1/2013<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
PALB<br />
PHARMACEUTIC<br />
ALS 12/1/2011<br />
259<br />
MARCH 1, 2011
2218 ZIMORGLOBIN<br />
HAEMOGLOBIN+IR<br />
ON(III)HYDROXY<br />
POLYMALTOSE+VI<br />
TAMIN B12<br />
2219 ZINACEF CEFUROXIME 750 mg<br />
2220 ZINC OXIDE ZINC OXIDE 15%w/w<br />
2221 ZINCOCET<br />
2222 ZINCOFER LIQUID<br />
2223 ZINCOLAC B<br />
2224 ZINCOVIT<br />
2225 ZINCOVIT<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
100 mg+60<br />
mg+16 mcg Syrup<br />
Injectable<br />
Powder<br />
AMMONIUM<br />
CHLORIDE+CETIRI<br />
ZINE+DEXTROMET<br />
HOPHAN+OTHERS multiple Syrup<br />
FOLIC<br />
ACID+HAEMOGLO<br />
BIN+IRON+VITAMI<br />
N B12+ZINC Multiple Syrup<br />
LACTOBACILLUS+<br />
MINERALS+VITAMI<br />
NS Multiple<br />
BODYCOTE<br />
PHARMACY<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE S. p.<br />
A. (Italy)<br />
Ointment<br />
Topical DANNEX LTD<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN Multiple Syrup<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN Multiple Tablet Oral<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD<br />
GLAXOWELLCOME<br />
SOUTH AFRICA<br />
(PTY) LTD.<br />
BODYCOTE<br />
PHARMACY<br />
LIMITED 7/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 5/1/2012<br />
AYRTON <strong>DRUG</strong><br />
MFG. COMPANY<br />
LTD DANNEX LTD 10/1/2012<br />
ALKEM ALKEM<br />
LABORATORIE LABORATORIES<br />
S LIMITED LIMITED<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
APEX<br />
LABORATORIES<br />
LTD<br />
FAR EAST<br />
MERCANTILE 11/1/2012<br />
JEFF PHARMACY<br />
LTD 12/31/2011<br />
FOURRTS<br />
(INDIA)<br />
LABORATORIE FOURRTS (INDIA)<br />
S<br />
LABORATORIES 10/1/2011<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
APEX<br />
LABORATORIES<br />
LTD<br />
APEX<br />
LABORATORIES<br />
LTD<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 7/1/2013<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2011<br />
260<br />
MARCH 1, 2011
2226 ZINCOVIT<br />
GINSENG+MINERA<br />
LS+MULTIVITAMIN Multiple Oral Drops<br />
2227 ZINEL PARACETAMOL 120 mgl5mls<br />
2228 ZINNAT CEFUROXIME 125 mg/5 mls<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Oral<br />
Suspension<br />
Oral<br />
Suspension<br />
2229 ZINNAT CEFUROXIME 250 mg Tablet Oral<br />
2230 ZINNAT CEFUROXIME 500 mg Tablet Oral<br />
2231 ZINNAT CEFUROXIME 125 mg Tablet Oral<br />
2232 ZINVITE<br />
2233 ZIPFERON<br />
2234 ZIPFERON<br />
INDUS<br />
PHARMACEUT<br />
ICALS<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUT<br />
ICAL LTD<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
GLAXOSMITH<br />
KLINE UK<br />
LIMITED<br />
APEX<br />
LABORATORIES<br />
LTD<br />
AMPONSAH EFFAH<br />
PHARMACEUTICAL<br />
LTD<br />
MEDREICH<br />
LIMITED<br />
MEDREICH<br />
LIMITED<br />
MEDREICH<br />
LIMITED<br />
MEDREICH<br />
LIMITED<br />
IRON+MINERALS+<br />
MULTIVITAMINS Multiple Syrup GVS LABS GVS LABS<br />
AMINO<br />
ACIDS+FOLIC<br />
ACID+IRON+VITB.<br />
12 Multiple Syrup<br />
IRON+MINERALS+<br />
MULTIVITAMINS Multiple<br />
Capsule oral<br />
(Hard<br />
gelatin)<br />
GB PHARMA<br />
(GH) LTD<br />
GB PHARMA<br />
(uk) LTD.<br />
MISSION VIVACARE<br />
LIMITED<br />
MISSION VIVACARE<br />
LIMITED<br />
M & G<br />
PHARMACEUTIC<br />
ALS LTD 12/31/2011<br />
AMPONSAH<br />
EFFAH<br />
PHARMACEUTIC<br />
AL LTD 12/31/2012<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 11/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
GLAXOSMITHKLI<br />
NE GHANA<br />
OFFICE 4/1/2011<br />
TOBINCO<br />
PHARMACY 8/1/2011<br />
GB PHARMA<br />
(GH) LTD 10/1/2012<br />
GB PHARMA<br />
(GH) LTD 5/1/2013<br />
261<br />
MARCH 1, 2011
2235 ZITHROMAX AZITHROMYCIN 500 mg/vial<br />
2236 ZMAX S.R. AZITHROMYCIN 2g/Bottle<br />
2237 ZOLADEX GOSERELIN 3.6 mg<br />
2238 ZOLADEX LA GOSERELIN 10.8 mg<br />
2239 ZOPERCIN<br />
2240<br />
ZUBES CHILDRENS<br />
COUGH<br />
2241 ZUBES EXPECTORANT<br />
2242 ZUBES EXTRA STRONG<br />
2243<br />
ZUBES EXTRA STRONG<br />
COUGH LOZENGES<br />
PIPERACILLIN+TA<br />
ZOBACTAM 4.0 g + 0.5 g<br />
IPECACUANHA+SQ<br />
UILL<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
Injectable<br />
Powder<br />
Powder for<br />
Prolonged<br />
Release oral<br />
Suspension<br />
Injectable<br />
Solution<br />
Injectable<br />
Solution<br />
Injectable<br />
Powder<br />
0.00119<br />
ml/0.1124<br />
ml/5 mls Syrup<br />
AMMONIUM<br />
CHLORIDE+DIPHE<br />
NHYDRAMINE Multiple Syrup<br />
ANISEED<br />
OIL+MENTHOL+PE<br />
PPERMINT OIL Multiple Mixtures<br />
EUCALYPTUS<br />
OIL+MENTHOL+PE<br />
PPERMINT<br />
OIL+OTHERS Multiple Lozenges<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
PFIZER<br />
GLOBAL<br />
PHARMACEUT<br />
ICALS<br />
(NIGERIA)<br />
ASTRAZENEC<br />
A UK LTD<br />
ASTRAZENEC<br />
A UK LTD<br />
ORCHID<br />
HEALTHCARE<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PZ CUSSONS<br />
GH. LTD<br />
PFIZER PGM<br />
(FRANCE)BEN<br />
VENUE<br />
LABORATORIES INC<br />
PFIZER<br />
PHARMACEUTICALS<br />
LLC (Puerto Rico)<br />
ASTRAZENECA UK<br />
LTD<br />
ASTRAZENECA UK<br />
LTD<br />
ORCHID<br />
HEALTHCARE<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PZ CUSSONS GH.<br />
LTD<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 4/1/2012<br />
PFIZER<br />
REGIONAL<br />
OFFICE (Ghana) 4/1/2012<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 5/1/2011<br />
ASTRAZENECA<br />
SCIENTIFIC<br />
OFFICE 6/1/2013<br />
EAST<br />
CANTONMENTS<br />
PHARMACY 4/1/2013<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
PZ CUSSONS<br />
GH. LTD 4/1/2011<br />
262<br />
MARCH 1, 2011
2244 ZUDREX<br />
CHLORPHENIRAMI<br />
NE+MENTHOL 2 mg + 1 mg<br />
+PARACETAMOL+S +250 mg + 60<br />
ODIUM CITRATE mg Syrup<br />
2245 ZYPREXA OLANZAPINE 5 mg Tablet Oral<br />
2246 ZYPREXA OLANZAPINE 10 mg Tablet Oral<br />
2247<br />
LIST OF <strong>REGISTER</strong>ED PRODUCTS<br />
GEO<br />
MEDICORE<br />
LIMITED<br />
GEO MEDICORE<br />
LIMITED GEO PHARMACY 8/1/2013<br />
ELI LILLY AND<br />
(suisse) SA (<br />
Kenya) LILY S.A.(Spain) REISS & CO. 12/1/2012<br />
ELI LILLY AND<br />
(suisse) SA (<br />
Kenya) LILY S.A.(Spain) REISS & CO. 12/1/2012<br />
263<br />
MARCH 1, 2011