FM SEPTEMBER 2018 ISSUE - digital edition
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
cover story<br />
CATH-MOUNTED HEART<br />
PUMPS FOR SHORT-TERM USE<br />
The Indian drug regulator granted approval to Impella heart pumps in July <strong>2018</strong><br />
for use during high-risk percutaneous coronary intervention<br />
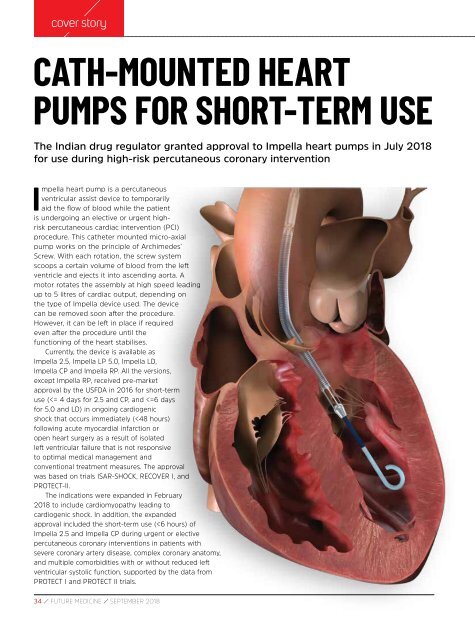
Impella heart pump is a percutaneous<br />
ventricular assist device to temporarily<br />
aid the flow of blood while the patient<br />
is undergoing an elective or urgent highrisk<br />
percutaneous cardiac intervention (PCI)<br />
procedure. This catheter mounted micro-axial<br />
pump works on the principle of Archimedes’<br />
Screw. With each rotation, the screw system<br />
scoops a certain volume of blood from the left<br />
ventricle and ejects it into ascending aorta. A<br />
motor rotates the assembly at high speed leading<br />
up to 5 litres of cardiac output, depending on<br />
the type of Impella device used. The device<br />
can be removed soon after the procedure.<br />
However, it can be left in place if required<br />
even after the procedure until the<br />
functioning of the heart stabilises.<br />
Currently, the device is available as<br />
Impella 2.5, Impella LP 5.0, Impella LD,<br />
Impella CP and Impella RP. All the versions,<br />
except Impella RP, received pre-market<br />
approval by the USFDA in 2016 for short-term<br />
use (