Cover Page Chemistry - Copy - Copy (2)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
By: ZHWAN REMEDAN YAAQUB | Group: B<br />
Duhok Polytechnic University<br />
College Of Technical Engineering<br />
Petrochemical Department<br />
Subject: Eng.chemistry ||<br />
Name Of Experiment: PROPERTIES OF ALCOHOLS: STRUCTURE, REACTIONS AND<br />
IDENTIFICATION OF ALCOHOLS<br />
Date: 2019/4/6
EXPERIMENT 3<br />
PROPERTIES OF ALCOHOLS: STRUCTURE, REACTIONS<br />
AND IDENTIFICATION OF ALCOHOLS<br />
Content:<br />
1-Objective.<br />
2-Introduction.<br />
3-Procedure.<br />
4-Discussion.<br />
5-Post-lab question.<br />
1-Objective<br />
a) observe some physical and chemical properties of selected alcohols.<br />
b) Relate the observed properties to the molecular structure. Identify an unknown<br />
alcohol.<br />
c) To learn some common properties of alcohols<br />
d) To distinguish phenols from the three types of alcohols by chemical tests<br />
2-Introduction<br />
Alcohols are organic compounds containing an -OH functional group bonded to a carbon atom.<br />
There are three classes (types) of alcohols: 1.primary, 2.secondary, 3.tertiary.<br />
A primary alcohol is an alcohol which has the hydroxyl group connected to<br />
a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH”<br />
group<br />
A secondary alcohol is a compound in which a hydroxy group, ‒OH, is attached to<br />
a saturated carbon atom which has two other carbon atoms attached to it<br />
A tertiary alcohol is a compound in which a hydroxy group, ‒OH, is attached to a<br />
saturated carbon atom which has three other carbon atoms attached to it.
Physical Properties<br />
Molecular State-Straight-chain alcohols with up to 12 carbon atoms are<br />
liquids.<br />
Solubility:Alcohols are soluble in water. This is due to the hydroxyl<br />
group in the alcohol which is able to form hydrogen bons<br />
with water molecules .<br />
The boiling point of alcohol depends on which type of alcohol you're<br />
using, as well as the atmospheric pressure. The boiling point<br />
decreases as atmospheric pressure decreases, so it will be slightly<br />
lower unless you are at sea level. Here is a look at the boiling point<br />
of different types of alcohol.<br />
The temperature that a solid substance becomes a liquid ,,, the<br />
melting point of alcohol is -114<br />
Viscosity refers to the resistance to flow.<br />
Chemical Properties of Alcohols:<br />
Alcohols are neutral compounds; the hydroxyl group does not ionize.<br />
However, the alcohol functional group is chemically reactive in other<br />
ways.<br />
Alcohol oxidation is an important organic reaction. Primary alcohols<br />
can be oxidized either to aldehydes or to carboxylic acids, while<br />
the oxidation of secondary alcohols normally terminates at the<br />
ketone stage. Tertiary alcohols are resistant to oxidation ,, such as<br />
K2Cr2O7<br />
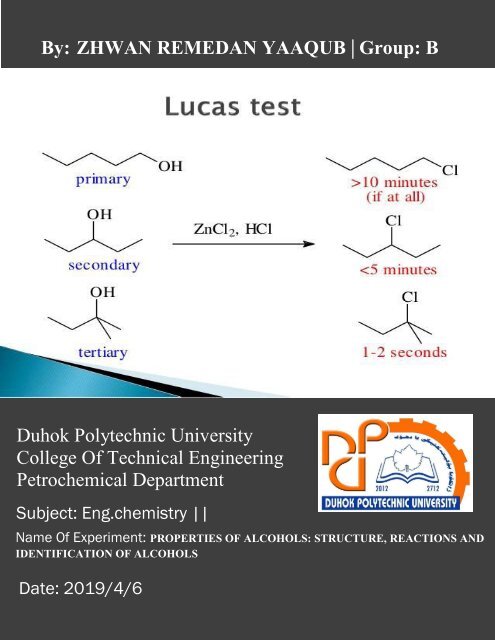
Lucas reagent is a solution of anhydrous zinc chloride (Lewis acid)<br />
in concentrated hydrochloric acid. It is used as<br />
a reagent to test alcohols and classify them in accordance to their<br />
reactivity.
3-Procedure.<br />
We have 2 method<br />
1. Oxidation.<br />
2. Lucas test.<br />
By Oxidation<br />
We will do this 3 steps in test tube<br />
1. We will prepare 5ml of K 2 Cr 2 O 7<br />
2. And 3 drop of H 2 SO 4<br />
3. 3 drop of propanol.<br />
The resulte is green<br />
By lucas test<br />
We will do this experiment in test tube<br />
1. We will prepare 5ml of K 2 Cr 2 O 7<br />
2. And 3 drop of H 2 SO 4<br />
3. 3 drop of Ethanol.<br />
Our result is Dark green<br />
The ethanol change color faster<br />
By lucas test<br />
We should do this also in test tube<br />
1. 2ml of Lucas<br />
2. 3 drop of 2-propanol<br />
Our result is white<br />
1. 2ml of Lucas<br />
2. 3 drop of Ethanol<br />
Our result is Very light yellow<br />
2-propanol will<br />
change color<br />
faster
4-Discussion