may edition file
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
₹ 250.00<br />
VOL 6 | ISSUE 1<br />
PAGES 100<br />
MAY 2019<br />
FUTUREMEDICINEINDIA.COM<br />
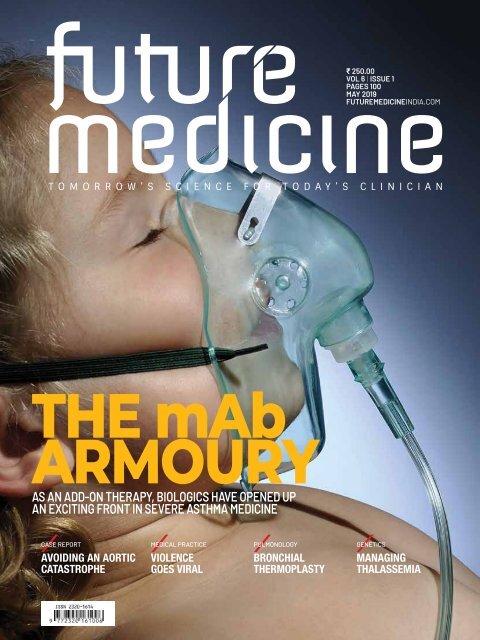
THE mAb<br />
ARMOURY<br />
AS AN ADD-ON THERAPY, BIOLOGICS HAVE OPENED UP<br />
AN EXCITING FRONT IN SEVERE ASTHMA MEDICINE<br />
CASE REPORT MEDICAL PRACTICE PULMONOLOGY GENETICS<br />
AVOIDING AN AORTIC<br />
CATASTROPHE<br />
VIOLENCE<br />
GOES VIRAL<br />
BRONCHIAL<br />
THERMOPLASTY<br />
MANAGING<br />
THALASSEMIA
editor’s note<br />
editor’s note<br />
Dear Doctor,<br />
May 2019 / Vol. 6 / Issue 1<br />
Founder AUGUST & 2018 Editor / Vol: 5 / Issue: 4<br />
CH Unnikrishnan<br />
Executive Editor<br />
S Harachand<br />
Science Editor<br />
Dr Rajanikant Vangala<br />
Consulting Editors<br />
Dr Founder Shivanee & Editor Shah<br />
Jeetha CH Unnikrishnan D’Silva<br />
Dr<br />
Executive<br />
Sumit<br />
Editor<br />
Ghoshal<br />
Copy S Harachand Editor<br />
Sreejiraj Eluvangal<br />
Science Editor<br />
Curator-cum-Correspondent<br />
Dr Rajanikant Vangala<br />
Divya Choyikutty<br />
Photo Copy Editor Editor<br />
Umesh Sreejiraj Eluvangal Goswami<br />
Design Consulting Editors<br />
Gopakumar Dr Shivanee Shah K<br />
Advisory Dr Sumit Ghoshal Board<br />
Dr<br />
Photo<br />
Devi<br />
Editor<br />
Shetty<br />
Dr B S Ajaikumar<br />
Umesh Goswami<br />
Dr Shashank Joshi<br />
Dr Illustrator Prof. Arumugam S<br />
Dr Mathewkutty I C Verma J Mattam<br />
Dr N K Warrier<br />
Dr Advisory Indira Board Hinduja<br />
Dr Devi Sekar Shetty Seshagiri<br />
Mr Dr B Rajesh S Ajaikumar R Nair<br />
Knowledge<br />
Dr Shashank Joshi<br />
Partner<br />
SGRF Dr Prof. Arumugam S<br />
Dr I C Varma<br />
National Business Head<br />
Shiny<br />
Dr N K Warrier<br />
Thomas<br />
Phone: Dr Sekar +91 Seshagiri 9821435120<br />
e-mail: Knowledge shiny@futuremedicineindia.com<br />
Partner<br />
Manager- SGRF Business Development<br />
Jyotsna Budhiraja<br />
Business Head<br />
Phone: +91 8586094582<br />
e-mail: Tushar Kanchan jyotsna@futuremedicineindia.com<br />
Chief Circulation Consultant & Subscription Manager<br />
(Circulation S Sanjeev Nair and Market Development)<br />
Rajesh<br />
Design &<br />
A<br />
Graphics<br />
Shah (MediaCafe)<br />
Phone: +91 9594 625 231<br />
e-mail:<br />
Blackboard<br />
rajesh@futuremedicineindia.com<br />
Kochi<br />
Circulation Editorial Offices Executive<br />
Sandesh BANGALOREChennamkulath<br />
Phone: Ground Floor, +91 9061969996<br />
JP Tower, Whitefield, Bangaluru.<br />
e-mail: MUMBAI subscribe@futuremedicineindia.com<br />
Editorial M9B, Press Enclave, OfficesPrateeksha Nagar, Sion East Mumbai.<br />
BANGALORE<br />
KOCHI<br />
Ground 3Rd Floor, Floor, Kurian JP Towers, Classic, Banerji Corporate Road Block,<br />
EPIP Ernakulam Zone, - 682 Whitefield, 018. Bengaluru - 560066<br />
MUMBAI<br />
Printed and Published by<br />
5 101, Wework Zenia, Central Circle,<br />
Ravi DeeCee, DC Books<br />
Hiranandani Business Park, Off. Ghodbunder Road,<br />
Thane, Printed at Mumbai, MH- 400 607.<br />
KOCHI Spenta Multimedia Pvt Ltd.<br />
3rd Lower Floor, Parel Kurian (W), Mumbai Towers, 400 013. Banerji Road<br />
Ernakulam - 682 018<br />
Printed and Published by<br />
Ravi The publishers DeeCee, regret DC that Books they cannot accept liability for errors or omissions<br />
Printed contained in at this publication, however caused. The opinions and views contained<br />
Millennium in this publication Arts, are not D-19/20, necessarily those Akurli of the Industrial publishers. Readers Estate, are advised<br />
Akurli to seek specialist Road, advice Kandivali before acting (East), on information Mumbai contained 400101, in this India publication,<br />
which is provided for general use and <strong>may</strong> not be appropriate for the readers’<br />
The<br />
particular<br />
publishers<br />
circumstances.<br />
regret that<br />
The<br />
they<br />
ownership<br />
cannot<br />
of<br />
accept<br />
trademarks<br />
liability<br />
is acknowledged.<br />
for errors or omissions<br />
No part of<br />
contained this publication in this or publication, any part of however the contents caused. thereof The opinions <strong>may</strong> be reproduced, and views contained stored in in a<br />
this retrieval publication system are or transmitted not necessarily in any those form of without the publishers. the permission Readers of are the advised publishers to<br />
seek in writing. specialist An exemption advice before is hereby acting granted on information extracts contained used for in the this purpose publication, of fair<br />
which is provided for general use and <strong>may</strong> not be appropriate for the readers’<br />
particular<br />
review.<br />
circumstances. The ownership of trademarks is acknowledged. No part of<br />
this publication or any part of the contents thereof <strong>may</strong> be reproduced, stored in a<br />
retrieval Printed and system Published or transmitted by Ravi in Dee any Cee, form DC without Books, the D C permission Kizhakkemuri of the Edam, publishers Good<br />
in Shephered writing. An Street, exemption Kottayam, is hereby Kerala granted on behalf for extracts of NextGen used Science for the purpose Media Pvt. of Ltd, fair<br />
review. printed at Spenta Multimedia Pvt, Lower Parel (West), Mumbai-400 013,India and<br />
Printed published and at Published DC Books, by D C Ravi Kizhakkemuri Dee Cee, DC Edam, Books, Good D C Shephered Kizhakkemuri Street, Edam, Kottayam, Good<br />
Shephered Street, Kottayam, Kerala on behalf of NextGen Science Media Pvt. Ltd,<br />
Kerala<br />
printed at Spenta Multimedia Pvt, Lower Parel (West), Mumbai-400 013,India and<br />
published at DC Books, D C Kizhakkemuri Edam, Good Shephered Street, Kottayam,<br />
Kerala © 2018 NextGen Science Media Pvt. Ltd, RNI Number KERENG/2012/44529<br />
© 2018 NextGen Science Media Pvt. Ltd, RNI Number KERENG/2012/44529<br />
This time, let me also invite your attention to a deserving cause in the context<br />
of a recently held conference of Undiagnosed Diseases Network International<br />
in Delhi. Dear Doctor The conference re-emphasised the need for more collaborative<br />
efforts from the clinical as well as the scientific community to investigate<br />
the We cause know of rare you are diseases. busy. There It is always has been reassuring an increased that the interest trust and among faith the of<br />
clinician hundreds community, of patients mainly your some healing research touch enthusiastic keeps you geneticists busy in this since noble most<br />
of these profession. conditions In the are hectic linked practice, to some it’s or quite other natural genetic that disorders, you might collecting miss<br />
valuable out on data some from of the real-world latest developments experience as well in emerging as dedicated medicine. researches. In this era<br />
But, of millions innovation, of patients medical suffering science from is getting such diseases redefined are almost still left by untreated the day. Old<br />
as technologies research hasn’t are yet being culminated replaced in to by development the new in the of blink effective of an treatment eye. Robots<br />
for and many artificial disorders intelligence or they are are not taking really over accessible a good to part a large of the section procedures, of the<br />
patient while population. genomics and This molecular should change. science unveil the mysteries of life further.<br />
Today’s We are need fortunate is for to the have young such and breakthroughs enthusiastic clinicians as they help from specialists across the like<br />
globe,<br />
you<br />
especially<br />
rise above<br />
India<br />
the expectations<br />
where the disease<br />
of today’s<br />
burden<br />
informed<br />
is huge,<br />
patient.<br />
to dedicate a<br />
fraction of their time to observe the patients who come with rare symptoms,<br />
and try to analyse the cause and take it forward with communities like UDNI.<br />
Similarly, it is also a time when India is witnessing revolutionary growth in<br />
Perhaps, you yourself can help provide a solution. As Dr I C Verma, the father<br />
healthcare industry, especially in the private sector, wherein an increasing<br />
of Indian medical genetics, advises in this <strong>edition</strong>’s Holy Grail, this is the age<br />
number of doctors are taking up multiple roles of clinician, researcher and<br />
of genomic medicine and do spend some time to learn this technology and<br />
entrepreneur. This requires expansion of your focus to a wider canvas. In<br />
try to interpret your findings in its light.<br />
this context, it becomes important how a busy professional like you can<br />
In many ways, this is applicable to even the age-old and established<br />
diseases<br />
keep pace<br />
as well,<br />
with<br />
as<br />
these<br />
highlighted<br />
latest<br />
in<br />
developments<br />
the cover story<br />
in a<br />
on<br />
quick<br />
asthma<br />
and<br />
—<br />
easy<br />
a<br />
way.<br />
well-recognised medical condition. The story extensively talks about the<br />
changing At Future concepts Medicine, of asthma which is since conceived recent studies and crafted observed by a that team there of senior is<br />
no journalists, one universal scientists definition and for doctors, this disease. our aim The is heterogeneity to help you do of just asthma that. We<br />
prompts are equipped us to look to at bring it more you as the an latest umbrella from term. the science of care from across<br />
In the Straight world Talk, in an Dr interesting Vijay Chandru, convenient founder of Strand way, supplemented Life Sciences, by calls the for best a<br />
renewed of views effort and to analyses from India’s the actual masters disease in each burden field. as We the present country you hasn’t this<br />
understood specialised its knowledge actual patient vehicle population, that plugs not only you into under the rare emerging diseases world but of<br />
also care widely seamlessly. prevalent Come, cancer-like let’s join afflictions. hands in this information journey.<br />
Happy CH Unnikrishnan<br />
reading<br />
editor@futuremedicineindia.com<br />
C H Unnikrishnan<br />
editor@futuremedicineindia.com<br />
www.futuremedicineindia.com futuremedicineindia FutureMedIndia<br />
AUGUST 2018/ FUTURE MEDICINE / 3
CASE REPORT MEDICAL PRACTICE PULMONOLOGY GENETICS<br />
Vol 6 Issue 1<br />
May 2019<br />
₹ 250.00<br />
VOL 6 | ISSUE 1<br />
PAGES 100<br />
MAY 2019<br />
FUTUREMEDICINEINDIA.COM<br />
THE mAb<br />
ATTACK<br />
40<br />
AS AN ADD-ON THERAPY, BIOLOGICS HAVE OPENED UP<br />
AN EXCITING FRONT IN SEVERE ASTHMA MEDICINE<br />
AVOIDING AN AORTIC<br />
CATASTROPHE<br />
VIOLENCE<br />
GOES VIRAL<br />
BRONCHIAL<br />
THERMOPLASTY<br />
MANAGING<br />
THALASSEMIA<br />
CASE REPORT<br />
ROBOTIC<br />
BYPASS<br />
REGULAR FEATURES<br />
06 Letters<br />
08 News updates<br />
30 Pulmonology<br />
32 Drug approvals<br />
52 Research snippets<br />
56 Hospital news<br />
62 Devices&gadgets<br />
72 Guidelines<br />
90 Events<br />
96 Calendar<br />
98 Holy grail<br />
Columns<br />
14 THE CATALYST<br />
Muralidharan Nair<br />
48 THE CELLVIEW<br />
Dr Rajani Kanth Vangala<br />
60 TRIALOMICS<br />
Dr Arun Bhatt<br />
50<br />
MEDICAL PRACTICE<br />
VIOLENCE<br />
GOES VIRAL<br />
Doctors are the most<br />
vulnerable when it comes<br />
to workplace violence<br />
36<br />
STRAIGHT TALK<br />
“WE STILL DON’T<br />
HAVE A CLEAR<br />
UNDERSTANDING<br />
OF THE ACTUAL<br />
DISEASE BURDEN<br />
IN INDIA”<br />
Vijay Chandru
58<br />
FROM THE INDUSTRY<br />
“OUR AIM IS TO HELP<br />
OPHTHALMOLOGISTS TO<br />
REMAIN STRAIN-FREE”<br />
12<br />
GENETICS<br />
ADVANCES IN<br />
MANAGING<br />
THALASSEMIA<br />
Several promising therapy<br />
regimens are underway to<br />
deal with the hereditary<br />
haemolytic disease<br />
68<br />
AMRITA CENTRE<br />
FOR ROBOTIC<br />
SURGERY TRAINING<br />
It’s important that<br />
we recognize that<br />
asthma treatment<br />
is not “one size<br />
fits all”,<br />
Andy Nish MD<br />
Fellow of American<br />
Academy of Allergy,<br />
Asthma &<br />
Immunology, USA<br />
16<br />
COVER STORY<br />
18<br />
COVER STORY<br />
UNLOCKING<br />
ASTHMA:<br />
MALARIA<br />
THE mAb WAY<br />
The emergence of biologics as<br />
RISEN an add-on therapy AGAIN?<br />
has opened<br />
up an exciting front in the new<br />
Global<br />
era of<br />
efforts<br />
severe<br />
to<br />
asthma<br />
contain<br />
medicine<br />
the<br />
curable disease suffers a<br />
backlash as cases shoot up in<br />
several regions
EDUCATION CASE REPORT GENETICS OPHTHALMOLOGY<br />
letters to the editor<br />
DIPLOMA TO<br />
DEGREE<br />
A DELICATE<br />
TRANSLOCATION<br />
GENETIC PATHWAYS<br />
TO TACKLE<br />
PLASMODIUM<br />
Better medium for<br />
getting updates<br />
₹ 250.00<br />
VOL 5 | ISSUE 12<br />
PAGES 100<br />
APRIL 2019<br />
FUTUREMEDICINEINDIA.COM<br />
MALARIA<br />
STRIKES BACK<br />
A STEADY SPURT IN NEW CASES WARNS OF A RESURGENCE<br />
OF A CURABLE DISEASE FROM NEAR ELIMINATION<br />
TIP OF AN<br />
‘EYES’BERG?<br />
Hello sir,<br />
I found this magazine from<br />
our medical college library<br />
3 months ago. As a medical<br />
student I am interested<br />
in knowing new updates<br />
in medical field and this<br />
magazine really helps to fulfill<br />
it. Special thanks to the editor<br />
for the same. Is there any<br />
special subscription offer for<br />
medical students?<br />
Lakshmi<br />
Medical Student<br />
Calicut<br />
Rich in content<br />
Dear editor,<br />
I have gone through the cover<br />
story regarding the topic<br />
Malaria published in April 19<br />
issue. It was very informative<br />
story and it covered all latest<br />
updates of the topic. Honestly<br />
great work by the editorial<br />
team.<br />
Dr. Vineeth<br />
UAE<br />
To be noticed more<br />
Hi,<br />
The article “Starting to forget”<br />
based on Alzheimer’s in<br />
February issue was really an<br />
important topic and it is to be<br />
delivered to all doctors as well<br />
as public. Lot of people in our<br />
society are suffering from this<br />
disease and not recognizing<br />
by their beloved people since<br />
they are considering it as<br />
geriatric problem.<br />
Dr. Jacob Mathews<br />
Hyderabad<br />
Hats off<br />
Dear sir,<br />
Great work by team Future<br />
Medicine. I just completed<br />
my reading at a stretch while<br />
traveling from Mumbai to<br />
Pune. The articles were fresh,<br />
catching and interesting. This is<br />
the a magazine we are looking<br />
for and hats off guys for the<br />
same.<br />
Dr. Hafiz Hamza<br />
Pune<br />
Guidance from medical<br />
experts<br />
Hi,<br />
I have been a subscriber of<br />
Future Medicine for the last 4<br />
months. The interviews with<br />
experienced doctors have<br />
helped me to understand the<br />
approach they following in<br />
various medical situations. I<br />
believe that experience makes<br />
a doctor an expert in it. Great<br />
work and keep it up.<br />
Dr. Praveen<br />
Kochi<br />
& GET 20 %<br />
NOW<br />
Please send me my subscription of FUTURE MEDICINE for (Select your plan)<br />
SUBSCRIBE<br />
One year Rs. 2,400/- Two years Rs. 4,800/- Three years Rs. 7,200/-<br />
OFF<br />
NAME<br />
ADDRESS<br />
CITY<br />
POSTAL CODE<br />
E-MAIL<br />
PHONE<br />
Fill complete details and send it along with the cheque/DD in favour of ‘NEXTGEN SCIENCE MEDIA (P.) LTD.’ to Future Medicine,<br />
5th floor, Wework Zenia, Zenia Building, Hiranandani Business Park, Off. Ghodbunder Road, Thane, Mumbai, MH- 400 607<br />
For NEFT/RTGS : Account No. 50200032001372, IFSC:- HDFC0000684 Name: NextGen Science Media Pvt Ltd, HDFC Bank Ltd,<br />
Kakkanad Branch, Cochin • For more details call - 9594 625 231 or mail - subscribe@futuremedicineindia.com
A medical science and news magazine for every new-age<br />
clinician. It empowers doctors with the most relevant updates,<br />
trends, case studies, expert views, knowledge exchange,<br />
hospital management and latest breakthroughs in medical<br />
science. To be relevant in the future of care, subscribe today.<br />
20%<br />
20%<br />
Rs. 2,400/-<br />
Rs. 4,800/-<br />
Rs. 7,200/-<br />
AUGUST 2018/ FUTURE MEDICINE / 59
news updates<br />
J&J donates<br />
10,000 more doses<br />
of bedaquiline<br />
to India<br />
Johnson & Johnson (J&J)<br />
will be providing an<br />
additional 10,000 courses of<br />
bedaquiline free of charge<br />
to Indian patients through<br />
US Agency for International<br />
Development (USAID), for<br />
a total of 20,000 donated<br />
courses.<br />
Earlier, 10, 000 courses<br />
of the drug to treat multidrug<br />
resistant TB have been<br />
supplied through Janssen<br />
Pharmaceutical Companies,<br />
J&J in 2016 as part of a<br />
global donation programme,<br />
operated in partnership with<br />
USAID under conditional<br />
access programme (CAP).<br />
“Ensuring access to<br />
bedaquiline in India is a top<br />
priority for J&J, given the<br />
country’s high burden of<br />
MDR-TB,” said Paul Stoffels,<br />
vice chair of the Executive<br />
Committee and chief scientific<br />
officer, Johnson & Johnson, in<br />
a statement.<br />
Following this, India<br />
will be able to procure<br />
bedaquiline via the Stop TB<br />
Partnership’s Global Drug<br />
Facility at J&J’s special-effort<br />
price of $400, announced in<br />
July 2018. This price enables<br />
More drug combos<br />
under scanner<br />
India <strong>may</strong> ban more fixed dose<br />
combinations (FDCs), currently available<br />
in the market, which are suspected to be<br />
`irrational’.<br />
The Drugs Technical Advisory Board<br />
(DTAB), which advises the drug regulator on<br />
safety issues of pharmaceutical products,<br />
has constituted a sub-committee to<br />
examine some 150-odd FDCs, reports said.<br />
The government slapped a ban on 344<br />
FDCs in 2016 following a report submitted<br />
by a panel led by Kokate, vice-chancellor of<br />
KLE University in Karnataka.<br />
Deliberating on a petition <strong>file</strong>d by drug<br />
companies challenging the government’s<br />
ban, the Supreme Court referred the matter<br />
to DTAB for a fresh review in December<br />
2017. The SC asked the board to check<br />
whether these drugs should continue to be<br />
sold.<br />
As per order, an expert panel under<br />
the chairmanship of Dr Kshirsagar<br />
reviewed the safety and efficacy of these<br />
drugs and recommended to continue the<br />
ban as the FDCs in question had no<br />
rational basis.<br />
the company to support<br />
manufacturing, distribution<br />
and surveillance programmes<br />
to safeguard the antibiotic’s<br />
effectiveness, he added.<br />
There are an estimated<br />
135,000 new cases of drugresistant<br />
TB in India every<br />
year, and currently less<br />
than 30% of these patients<br />
are diagnosed and put on<br />
an appropriate treatment<br />
regimen.<br />
The government of India<br />
has set the ambitious goal<br />
of ending TB by 2025, five<br />
years ahead of the global<br />
Sustainable Development Goal<br />
(SDG) target of 2030.<br />
India to regulate<br />
OPS as drugs<br />
India will regulate organ<br />
preservative solutions (OPS)<br />
as drugs, according to a new<br />
official notification.<br />
The government has<br />
included Organ Preservative<br />
Solutions (OPS) to the list of<br />
notified medical devices placing<br />
it under the purview of Section<br />
3 (b) (iv) of the Drugs and<br />
Cosmetics (D&C) Act 1940 with<br />
immediate effect.<br />
Section 3(b) of the Drugs<br />
and Cosmetics (D&C) Act 1940<br />
talks about “drugs” as inclusive<br />
of such devices intended for<br />
internal or external use in the<br />
diagnosis, treatment, mitigation<br />
or prevention of disease or<br />
disorder in human beings or<br />
animals.<br />
8 / FUTURE MEDICINE / May 2019
According to a Gazette<br />
notification, issued to this<br />
effect, the government<br />
specified OPS intended for<br />
external or internal use in<br />
human beings as drugs. OPS is<br />
a crucial medical device that is<br />
used to supply oxygen and vital<br />
nutrients to harvested organs,<br />
thereby increasing their life and<br />
maintaining their vitality.<br />
Drug regulator<br />
slaps safety<br />
alerts on<br />
antibiotics<br />
Drugs Standard Control<br />
Organisation (CDSCO)<br />
has ordered drug companies<br />
manufacturing certain<br />
commonly used antibiotics to<br />
carry safety warning on the<br />
labels of these medicines.<br />
The drug regulator wanted<br />
to ensure that this information<br />
is made available to the<br />
general public.<br />
The decision comes<br />
after the National Coordination<br />
Centre of<br />
the Pharmacovigilance<br />
Programme of India<br />
(PvPI) sounded caution on<br />
commonly used antibiotics<br />
including cefotaxime, ofloxacin<br />
and cefixime.<br />
PvPI reported the risk of<br />
developing Stevens-Johnson<br />
Syndrome and toxic epidermal<br />
necrolysis among people<br />
WARNING<br />
using the antibiotic ofloxacin.<br />
Injectable cefotaxime has<br />
been implicated for causing<br />
angioedema.<br />
The PvPI recommended<br />
that CDSCO take necessary<br />
steps to incorporate the<br />
adverse drug reactions in<br />
the prescribing leaflet of<br />
these drugs marketed in the<br />
country, reports said.<br />
The subject expert<br />
committee under the<br />
Drug Controller General<br />
India (DCGI) examined<br />
PvPI’s suggestions and<br />
recommended to incorporate<br />
safety cautions in its packages<br />
of all such antibiotics.<br />
Feeder<br />
qualification for<br />
super specialty<br />
courses revised<br />
The Medical Council<br />
of India has revised<br />
the feeder qualifications<br />
of six super specialty<br />
courses. Feeder<br />
qualification for 2 DM<br />
(Doctor of Medicine) and<br />
4 MCh courses (Master of<br />
Chirurgie) has been revised<br />
through an amendment<br />
to the older regulations<br />
Postgraduate Medical<br />
Education Regulations, 2000<br />
and are effective from April 1,<br />
2019.<br />
The council has removed<br />
many other redundant<br />
qualifications which act as<br />
prior requirements to various<br />
DM and MCh courses through<br />
a gazette notification.<br />
Following the amendment,<br />
candidates with MD in<br />
General Medicine or Radiation<br />
Oncology can now pursue<br />
DM (Medical Oncology). The<br />
prior requirements were MD<br />
(Medicine), MS (Radiotherapy),<br />
MD (Paediatrics). For DM<br />
(Interventional Radiology),<br />
earlier the feeder qualification<br />
was stated as MD (Radiology)<br />
and now the name has<br />
been changed to MD<br />
(Radiodiagnosis).<br />
Similarly, the candidate<br />
qualified with MS (General<br />
Foetus over 20 weeks can be aborted to<br />
save a life: Bombay HC<br />
Aregistered clinician<br />
can perform medical<br />
termination of pregnancy<br />
(MTP) on a foetus that has<br />
crossed the legal abortion<br />
limit of 20 weeks without<br />
the court’s permission if it is<br />
deemed necessary to save<br />
the life of the woman, the<br />
Bombay high court said in a<br />
recent ruling.<br />
The permission for MTP,<br />
however, still has to be<br />
sought when a pregnancy<br />
has exceeded 20 weeks<br />
and the woman “fears its<br />
continuation would involve<br />
grave injury to her physical or<br />
mental health or where there<br />
is a substantial risk that if<br />
the child were born, it would<br />
suffer from such physical or<br />
mental abnormalities as to be<br />
seriously handicapped”.<br />
In such cases, the<br />
pregnant woman will have<br />
to seek permission from the<br />
high court and unless such<br />
permission is granted, no<br />
registered medical practitioner<br />
can terminate such pregnancy,<br />
the HC ruled.<br />
The court directed the<br />
state government to establish<br />
medical boards in every<br />
district to examine pregnant<br />
women and to furnish reports<br />
in cases where women<br />
have approached courts for<br />
permission after 20 weeks<br />
of gestation, within three<br />
months, reports said.<br />
May 2019 / FUTURE MEDICINE / 9
Surgery) can now go for MCh<br />
Surgical Oncology. Earlier<br />
the prior requirements were<br />
MS (Surgery), MS (ENT), MS<br />
(Orthopaedics), MD (Obst.<br />
& Gyn). Surgeons with MS<br />
(General Surgery) can apply<br />
for MCh (Head & Neck<br />
Surgery). Earlier they were<br />
MS (ENT) or MS (General<br />
Surgery) or MCh (Plastic &<br />
Reconstructive Surgery) or<br />
MCh (Surgical Oncology) or<br />
MCh (Neuro Surgery).<br />
Generic eribulin<br />
launched in India<br />
Ageneric version of the anticancer<br />
drug eribulin, which<br />
is currently marketed under<br />
the brand name Halaven by<br />
Eisai Pharmaceuticals, is now<br />
available in India.<br />
The generic eribulin will be<br />
40% cheaper than Halavan,<br />
said Emcure Pharmaceuticals,<br />
which launched the cancer<br />
treatment.<br />
Eribulin, originally isolated<br />
halichondrin B from the<br />
natural Japanese marine<br />
sponge Halichondria okadai,<br />
was approved by the US FDA<br />
on November 15, 2010 for the<br />
treatment of metastatic breast<br />
cancer.<br />
Considered less toxic,<br />
eribulin is currently used as<br />
second-line treatment for<br />
relapsing triple-negative breast<br />
cancer. Triple-negative breast<br />
cancer is tough to treat as the<br />
patients with this sub-type<br />
are tested negative for all<br />
the three receptor proteins —<br />
oestrogen, progesterone as<br />
well as HER2.<br />
Eisai won the second<br />
approval from the US FDA for<br />
eribulin in January 2016 or the<br />
treatment of liposarcoma in<br />
patients who had undergone<br />
anthracycline-based<br />
chemotherapy.<br />
Presently, eribulin is being<br />
investigated for use in various<br />
cancers such as non-small cell<br />
lung cancer, prostate cancer<br />
and sarcoma.<br />
Measles cases<br />
shoot up<br />
globally: WHO<br />
Reported cases of measles<br />
rose by 300 per cent<br />
Stop Thal to prevent thalassemia<br />
Kanakia Health Care,<br />
Mumbai, has created a<br />
program called STOP THAL<br />
under the guidance of Dr<br />
Swati Kanakia, a paediatric<br />
haemato-oncologist with<br />
a PhD in Thalassemia from<br />
Mumbai University. STOP<br />
THAL, Screening<br />
for Thalassemia and Opting<br />
for PREVENTION, is aimed<br />
at preventing thalassemia<br />
major cases in an effort<br />
to reduce the disease<br />
burden. Dr Kanakia firmly<br />
believes that the only way<br />
to reduce thalassemia<br />
major cases is by<br />
prevention.<br />
However, how does<br />
one suspect thalassemia?<br />
The “Magic Mantra” for<br />
considering thalassemia<br />
minor is low or normal<br />
hemoglobin, high RBC<br />
count, microcytosis and<br />
a normal RDW. This is<br />
followed up by hemoglobin<br />
electrophoresis, which<br />
detects only beta<br />
thalassemia. If there is<br />
a strong suspicion of<br />
thalassemia despite a<br />
normal Hb electrophoresis,<br />
mutations for the alpha<br />
thalassemia gene <strong>may</strong> be<br />
carried out to clinch the<br />
diagnosis.<br />
Each and every doctor<br />
can play a pivotal role by<br />
considering thalassemia<br />
on a regular CBC report<br />
done for any reason.<br />
Dr Kanakia hopes that<br />
STOP THAL will prove<br />
to be useful in reducing<br />
thalassemia major cases.<br />
The program, available at<br />
http://kanakiahealthcare.<br />
com/stopthal/, helps one<br />
to estimate the chances<br />
of having a completely<br />
normal, thalassemia minor,<br />
or thalassemia major child.<br />
It will be an important<br />
asset to increase awareness<br />
amongst thalassemia<br />
patients, and doctors <strong>may</strong><br />
use it as an aid for patient<br />
education.<br />
in the first three months<br />
of 2019 compared to the<br />
same period in 2018, shows<br />
preliminary global data by<br />
WHO.<br />
This follows consecutive<br />
increases over the past two<br />
years.<br />
Measles, a highly<br />
contagious airborne viral<br />
infection, can be entirely<br />
prevented through a twodose<br />
vaccine. But vaccination<br />
rates have been slipping in<br />
recent months.<br />
Global coverage with<br />
the first dose of measles<br />
vaccine has stalled at 85<br />
percent for several years.<br />
This is still short of the 95<br />
percent needed to prevent<br />
outbreaks, and leaves<br />
many people in many<br />
communities at risk. Second<br />
dose coverage, while<br />
increasing, stands at 67<br />
percent.<br />
“While this data is<br />
provisional and not yet<br />
complete, it indicates a<br />
clear trend. Many countries<br />
are in the midst of sizeable<br />
measles outbreaks, with<br />
all regions of the world<br />
experiencing sustained rises<br />
in cases,” WHO said in a<br />
statement.<br />
Current outbreaks<br />
include the Democratic<br />
Republic of the Congo,<br />
Ethiopia, Georgia, Kazakhstan,<br />
Kyrgyzstan, Madagascar,<br />
Myanmar, Philippines,<br />
Sudan, Thailand and Ukraine,<br />
causing many deaths –<br />
mostly among young<br />
children.<br />
Over recent months,<br />
spikes in case numbers<br />
have also occurred in<br />
countries with high overall<br />
vaccination coverage,<br />
including the United States<br />
of America as well as Israel,<br />
Thailand, and Tunisia. The<br />
agency noted that only<br />
about one in 10 actual<br />
measles cases are reported,<br />
meaning the early<br />
trends for 2019 likely<br />
underestimate the severity<br />
of the outbreaks.<br />
10 May 2019
Transvaginal mesh banned in US<br />
amid safety concerns<br />
The US Food and Drug<br />
Administration ordered the<br />
manufacturers of all remaining<br />
surgical mesh products<br />
indicated for the transvaginal<br />
repair of pelvic organ prolapse<br />
(POP) to stop selling and<br />
distributing their products<br />
immediately. The order is the<br />
latest in a series of escalating<br />
safety actions related to<br />
protecting the health of the<br />
thousands of women each<br />
year who undergo surgery<br />
transvaginally to repair POP.<br />
The FDA has determined<br />
that the manufacturers, Boston<br />
Scientific and Coloplast, have<br />
not demonstrated a reasonable<br />
assurance of safety and<br />
effectiveness for these devices,<br />
which is the premarket review<br />
standard that now applies<br />
to them since the agency<br />
reclassified them in class III<br />
(high risk) in 2016.<br />
As part of the 2016<br />
reclassification, manufacturers<br />
were required to submit and<br />
obtain approval of<br />
premarket approval (PMA)<br />
applications, the agency’s<br />
most stringent device review<br />
pathway, in order to continue<br />
marketing their devices in the<br />
US.<br />
“In order for these mesh<br />
devices to stay on the market,<br />
we determined that we<br />
needed evidence that they<br />
worked better than surgery<br />
without the use of mesh to<br />
repair POP. That evidence was<br />
lacking in these premarket<br />
applications, and we couldn’t<br />
assure women that these<br />
devices were safe and effective<br />
long term,” said Jeffrey Shuren,<br />
MD, director of the FDA’s Center<br />
for Devices and Radiological<br />
Health.<br />
In 2002, the first mesh<br />
device for transvaginal repair<br />
of POP was cleared for use<br />
as a class II moderate-risk<br />
device. About 1 in 8 women<br />
has surgery to repair POP over<br />
her lifetime, and a subset of<br />
these surgeries are completed<br />
transvaginally with the use of<br />
surgical mesh.<br />
Two manufacturers<br />
have been marketing three<br />
surgical mesh products for<br />
transvaginal repair of POP. In<br />
reviewing the PMAs submitted<br />
by the two manufacturers,<br />
the agency determined they<br />
failed to provide an adequate<br />
assessment of the long-term<br />
safety of these devices and<br />
failed to demonstrate an<br />
acceptable long-term benefit<br />
of these devices compared<br />
to transvaginal surgical tissue<br />
repair without the use of mesh,<br />
the agency said in a press<br />
statement.<br />
InARI signs MoU with NTTE Med<br />
University on translational research<br />
The present translational<br />
research in India will<br />
not be able to achieve its<br />
objectives unless important<br />
changes in education<br />
and training modules<br />
are addressed. The T1<br />
(bench to bedside) and T2<br />
(bedside to bench) phases<br />
of translational research<br />
are complementary and<br />
need important resources<br />
— mostly financial — that<br />
require quality collaborations<br />
between clinical and<br />
molecular scientists. It is<br />
important to note that in<br />
India, a lot of emphasis was<br />
given to develop physicianscientists<br />
(T2) and despite<br />
all the euphoria, innovations<br />
in the biomedical field have<br />
been negligible. The T1 cycle<br />
that exploits the expertise of<br />
research scientists outside<br />
medical institutes like<br />
universities and academic<br />
institutions has not been well<br />
integrated. It is extremely<br />
important to link molecular<br />
networks with clinical<br />
observations to complete<br />
the T1-T2 cycle. Especially<br />
in a country as big as ours<br />
with our low investment in<br />
research, more emphasis<br />
must be given to biomedical<br />
related problems where the<br />
scientific community works<br />
in tandem with clinicians. In<br />
this direction, the Institute<br />
for Applied Research and<br />
Innovation (InARI) and NITTE<br />
Medical University have<br />
joined hands by signing<br />
an MoU to create training,<br />
education and research<br />
programmes suitable for<br />
Indian healthcare needs.<br />
InARI focuses on applied<br />
research with direct benefits<br />
to the society and healthcare<br />
is its top priority. By signing<br />
this MoU with NITTE Medical<br />
University, a premier medical<br />
institute, a new chapter<br />
has begun in translational<br />
research for India<br />
Prof Dr Satheesh Kumar Bhandary (Vice Chancellor of NITTE Medical<br />
University), Dr Rajani Kanth Vangala (Founder and Managing Trustee<br />
of InARI), Dr Poornima V (co-founder and trustee – InARI), Dr Suchetha<br />
Kumari (Head of Research, NITTE Medical University)<br />
May 2019 / FUTURE MEDICINE / 11
genetics<br />
ADVANCES IN MANAGING<br />
THALASSEMIA<br />
Several promising therapy regimens are underway to deal with the<br />
hereditary haemolytic disease<br />
DR RAJANI KANTH VANGALA<br />
Thalassemia comes from two Greek<br />
words “Thalassa” and “emia”<br />
meaning “sea” and “blood”. This<br />
naming has a unique meaning as the<br />
disease affects the hemoglobin and<br />
was described almost 90 years ago<br />
by Cooley and Lee. Two gene clusters<br />
controlling haemoglobin synthesis are<br />
located on chromosome 16 (α-like<br />
globins) and chromosome 11 (β-like<br />
globins) in such a manner that they<br />
are differentially expressed at different<br />
stages of development like embryonic,<br />
foetal and adult. This is seen in<br />
β-globin gene cluster where, ε-gene is<br />
only expressed in early embryos and<br />
two γ-genes, which are expressed<br />
during gestation, are found in foetal<br />
haemoglobin (Hb F, α2γ2). The δ-gene<br />
used in diagnosing thalassemias is a<br />
component of Hb A2 (α2δ2), whereas<br />
α and β-globins combine to form<br />
major haemoglobin component Hb<br />
A (α2β2) carried by adult red blood<br />
cells. The heritable mutations in α and<br />
β gene clusters in thalassemias results<br />
in defective haemoglobin which binds<br />
to less oxygen and also reduces the<br />
transport of oxygen.<br />
There are three types of<br />
β-thalassemia, based on the β-globin<br />
chain imbalance and severity of<br />
anaemia; namely minor, intermedia and<br />
major. Several mutations have been<br />
identified and were classified into silent<br />
— which have no effect, mild — leading<br />
to a reduction in β-globin production<br />
levels, and severe — causing a complete<br />
absence of the β-globin gene product.<br />
The minor trait or carrier patients have<br />
heterozygous inheritance of mutations<br />
and are often clinically asymptomatic.<br />
Patients with the major trait have severe<br />
anaemia from infancy and become<br />
life-long dependents on transfusion.<br />
However, β –thalassemia intermedia has<br />
variable anaemia of mild to moderate<br />
requiring variable transfusions.<br />
β-thalassemia major and intermedia<br />
THE CLASSIFICATION<br />
OF α-THALASSEMIA IS<br />
DEPENDENT ON HOW THE<br />
TWO α-GLOBIN GENES ARE<br />
DELETED OR REDUCED IN<br />
ACTIVITY DUE TO MUTATIONS<br />
can be due to several homozygous or<br />
compound heterozygous inheritances<br />
of mutations in β-globin gene. Different<br />
modifications like the extent of<br />
α-globin to β-globin chain imbalance,<br />
ineffective erythropoiesis and the<br />
severity of anaemia cause β-thalessemia<br />
intermedia, rather than β-thalessemia<br />
major in most patients. Most frequent<br />
are mutations in the β-globin<br />
gene, second are co-inheritance of<br />
α-thalassemia, higher levels of γ-chains<br />
of globin and sustained production of<br />
foetal haemoglobin after infancy. The<br />
last factor — hereditary persistence of<br />
foetal haemoglobin — can be due to<br />
several rare mutations like deletions of<br />
upstream promoter or regulatory region<br />
but the presence of an intact gene,<br />
and the complete deletion of δ-globin<br />
and β-globin genes. These patients<br />
have one intact γ-globin gene which<br />
is called δβ-thalassemia. Some of the<br />
other complications, such as dominant<br />
inclusion body β-thalassemia, have a<br />
triplicated or quadruplicated α-genotype<br />
along with β-heterozygosity or E/<br />
β-thalassemia where β-thalassemia is<br />
co-inherited with a structural variant of<br />
hemoglobin E.<br />
Clinical categories<br />
The classification of α-thalassemia<br />
is dependent on how both the α-globin<br />
genes are deleted or reduced in activity<br />
due to mutations. The first group of<br />
α+-thalassemias are of several types,<br />
but are dominated by –α3.7 and –α4.2<br />
that correspond to the lengths of<br />
deletion in the α-globin gene. The other<br />
α+-thalassemias have several point<br />
mutations, the most common being<br />
chain-termination mutant haemoglobin<br />
Constant Spring called αCSα. The second<br />
group α0-thalassemias are due to<br />
deletion of both the α-globin genes (-/-<br />
), which might occur in heterozygous<br />
condition with α+-thalassemias (-α/- or<br />
αCSα/-). The ATR-16 syndrome, which<br />
involves α-globin gene on chromosome<br />
16, was shown to be associated<br />
with mental retardation, also called<br />
α-thalassemia x-linked intellectual<br />
disability (ATRX).<br />
The clinical categorization of<br />
thalassemias is being simplified based<br />
on clinical-management criteria. As<br />
transfusion remains the major form of<br />
12 / FUTURE MEDICINE / May 2019
therapy, the patients are divided into<br />
transfusion-dependent thalassemia<br />
(TDT) and non-transfusion-dependent<br />
thalassemia (NTDT). The NTDT <strong>may</strong><br />
still need a transfusion but not at the<br />
same rate as TDT, primarily for the<br />
prevention or management of certain<br />
diseases. Patients with β-thalassemia<br />
intermedia, haemoglobin H and<br />
moderate forms of haemoglobin E/βthalassemia<br />
usually constitute NTDT. The<br />
TDT patients have β-thalassemia major<br />
and severe forms of haemoglobin E/βthalassemia.<br />
Suppressing the abnormal<br />
erythropoiesis by transfusion can control<br />
downstream pathological mechanisms<br />
in thalassemia. Some of the long-term<br />
follow-up studies which evaluated birth<br />
cohorts of patients with β-thalassemia<br />
major found that transfusion along<br />
with iron-chelation improves survival.<br />
Transfusion therapy, however, does<br />
have the risk of secondary-iron overload<br />
as the human body does not have a<br />
means to excrete iron. Patients receiving<br />
2-4 units of blood per month will have<br />
5,000-10,000 mg of iron per year. This<br />
leads to more iron than the capacity<br />
of transferrin, thus causing hepatic<br />
and extrahepatic tissue damage. The<br />
extra non transferrin-bound iron also<br />
can transport into cardiomyocytes,<br />
hepatocytes, pancreatic β cells and<br />
anterior pituitary cells, generating<br />
reactive-oxygen species damaging<br />
subcellular organelles.<br />
Controlling iron overload<br />
The iron overload warrants prompt<br />
diagnosis in NTDT as it can cause<br />
substantial morbidity and mortality and<br />
in patients with TDT, excess iron can<br />
be seen as early as 2-6 years of age.<br />
Cardiomyopathy has been observed<br />
as a leading cause of mortality in<br />
TDT patients, whereas osteoporosis,<br />
thrombosis, pulmonary hypertension,<br />
silent strokes, hypogonadism,<br />
hypothyroidism and renal diseases<br />
are strongly associated with NTDT<br />
iron overload. Of the several methods,<br />
serum ferritin assessment is commonly<br />
used to indicate the need for initiation<br />
of iron-chelation therapy, for example<br />
in TD. Maintaining concentrations<br />
lower than 1000µg/L of ferritin leads<br />
to better-sustained therapy. Serum<br />
ferritin concentrations above 2500<br />
µg/L are strongly associated with an<br />
increased risk of cardiac and other<br />
diseases. Superconducting Quantum<br />
Interference Device (SQUID) is one of<br />
the important technologies to assess<br />
liver iron concentration, but is not widely<br />
used. Magnetic Resonance Imaging<br />
(MRI) has become the best alternative<br />
for non-invasive liver iron concentration<br />
evaluation. There are three iron chelators<br />
currently available for iron overload<br />
namely deferoxamine, deferasirox and<br />
deferiprone.<br />
Traditionally, splenectomy is<br />
performed as an alternative or as<br />
an adjunct to transfusion therapy.<br />
However, it has become obsolete in<br />
patients with TDT due to potential<br />
infections, but is used in NTDT. In<br />
patients with β-thalessemia intermedia,<br />
there is an increased risk of long-term<br />
thrombosis along with end-organ<br />
damage as the spleen also acts as<br />
a reservoir of toxic iron. Presently,<br />
splenectomy is recommended only<br />
for patients who are unable to receive<br />
transfusion and iron-chelating therapy<br />
and also have clinical symptoms of<br />
splenomegaly or hypersplenism.<br />
One of the best cures available for<br />
thalassemia with more than 80%<br />
disease-free survival is the replacement<br />
of mutant haematopoietic cells with<br />
haematopoietic stem cells when<br />
matched sibling donors are available.<br />
Further improvements in the graftversus-host<br />
disease and inducing<br />
graft tolerance have resulted in more<br />
unrelated donors with overall survival of<br />
65%. These improvements are showing<br />
that more novel therapies are in good<br />
progress and might result in better<br />
treatment regimes for thalassemia.<br />
The author is medical scientist and former<br />
director of SGRF, Bangalore<br />
May 2019 / FUTURE MEDICINE / 13
column<br />
the catalyst<br />
Bridging the trust deficit<br />
Time for private players to think in tandem with<br />
policy directions<br />
MURALIDHARAN NAIR<br />
The private sector healthcare delivery<br />
in India has been the bedrock of<br />
healthcare capability and capacity,<br />
particularly in the area of high-end<br />
secondary, tertiary and quaternary care.<br />
Multi-specialty, corporate hospital chains<br />
have been the flag bearers of capacity and<br />
capability growth in high-end care over the<br />
last decade or more, but they are currently<br />
struggling with the changing dynamics of<br />
healthcare business, which is rendering<br />
traditional business models suboptimal.<br />
While private sector promoters and investors<br />
see the changing dynamics characterised<br />
by increasing focus on affordability as an<br />
overbearing development, in my view, this<br />
was imminent, if not long overdue. The<br />
reason why this was possibly missed by the<br />
leaders and managers could be traced to the<br />
key tenets of their belief system. In essence, I<br />
have observed three, distinct tenets:<br />
Relative affordability compared to<br />
international prices: This has been an<br />
overwhelming influence in the minds of<br />
leaders of the private healthcare industry<br />
in India and has been a real deterrent in<br />
facilitating the requisite focus on affordable<br />
care in India. I could never understand the<br />
rationale behind this belief, as it has very<br />
little practical relevance for the consumer or<br />
payor for whom the affordability is defined<br />
by the size of their own pocket not by what<br />
would have been the relative burden in a<br />
hypothetical situation of being in a foreign<br />
land. This has singularly contributed in<br />
catalysing a self-righteous attitude among<br />
healthcare managers and leaders, which<br />
manifested in the use of price increases<br />
as the key tool for realising commercial<br />
objectives, instead of focusing on efficiency<br />
and the evolution of an operating<br />
model that responds effectively to the<br />
consumer context.<br />
Inordinate focus on experience: The quest<br />
to offer a differentiated experience from<br />
public healthcare facility is understandable,<br />
but in the course of time, this has stretched<br />
beyond a limit, which has not only resulted<br />
in higher capital and operations cost, but<br />
has given a perception of insensitivity to the<br />
large masses of population who were neither<br />
used to that kind of experience nor were<br />
seeking it, but had little or no option but to<br />
avail them in the absence of affordable and<br />
credible alternatives. In some ways, what is<br />
perhaps a welcome offering for a minuscule<br />
percentage of the population with the means<br />
is being perceived as vulgar compulsion by<br />
the masses. Barring some extreme exceptions,<br />
most of the time, the experience factors do<br />
not contribute as significantly to the overall<br />
cost of delivery when compared to the<br />
perception it creates of expenditure that the<br />
poor consumer perceives as an avoidable<br />
burden.<br />
Making healthcare affordable is<br />
government’s responsibility, not ours:<br />
This tenet has a rational basis, without doubt,<br />
and it is also true that the government has<br />
failed miserably to offer a credible alternative<br />
through public health delivery systems or<br />
to facilitate a robust ecosystem for health<br />
education to ensure adequate supply of<br />
clinical talent, particularly at the specialist<br />
level. This has significantly contributed to<br />
the excessive use of private care as well as<br />
the high cost of private care. But the irony is<br />
that the private sector was happy until the<br />
government started taking responsibility for<br />
affordable care and is now unhappy when<br />
it is pursuing the same with great intensity.<br />
That’s where the private sector erred, in<br />
assuming that government ownership of<br />
14 / FUTURE MEDICINE / May 2019
affordable healthcare will come on its terms,<br />
with the government playing the role of<br />
the payor, and will help them expand their<br />
market without changing their character. In<br />
reality, government ownership will expand<br />
the market significantly, but can find its<br />
sustainability only by changing the economics<br />
significantly to match its fiscal capacity, given<br />
the large percentage of needy population<br />
(approximately 70 percent).<br />
In conclusion, it is a fact that the vision of<br />
corporate healthcare chains (and even many<br />
of the trust hospitals whose commercial<br />
agenda seem no weaker than those of<br />
“for profit” healthcare enterprises) for their<br />
enterprise was not really in tandem with<br />
the needs of the vast majority of India’s<br />
population, even though they have played<br />
a stellar role in providing quality healthcare<br />
to multitudes of needy customers both rich<br />
and poor. However, it is equally true that<br />
government policies should target<br />
the good of the greatest population<br />
THE PRIVATE SECTOR WAS HAPPY<br />
UNTIL THE GOVERNMENT STARTED<br />
TAKING RESPONSIBILITY FOR<br />
AFFORDABLE CARE AND IS NOW<br />
UNHAPPY WHEN IT IS PURSUING<br />
THE SAME WITH GREAT INTENSITY<br />
and hence it is imperative that private<br />
healthcare providers must not waste time<br />
in defending status quo, but put in all their<br />
managerial, financial and entrepreneurial<br />
might to redefine their vision for the future in<br />
a way that is in harmony with the needs of<br />
the larger chunks of the population as well<br />
as the policy direction. This will, in fact, go<br />
a long way in bridging the significant trust<br />
deficit that exists between private players<br />
and policymakers, leading to better mutual<br />
empathy, and consequently, more effective<br />
policies, principles and practices for the<br />
benefit of all.<br />
The author has long-standing association with<br />
EY India but the views are strictly personal.<br />
May 2019 / FUTURE MEDICINE / 15
cover story<br />
UNLOCKING<br />
16 / FUTURE MEDICINE / May 2019
ASTHMA<br />
THE<br />
mAb WAY<br />
The emergence of biologics as an<br />
add-on therapy has opened up an<br />
exciting front in the new era of<br />
severe asthma treatment<br />
S HARACHAND<br />
Asthma is a heterogeneous disease, characterised by<br />
inflammation of the air passages of the lungs.<br />
World over, an estimated 235 million people suffer<br />
from asthma, a chronic condition characterised by symptoms<br />
such as difficulty in breathing, tightness in the chest and<br />
wheezing.<br />
Caused by a combination of complex environmental<br />
and genetic interactions, the underlying mechanisms of this<br />
airway disorder is yet to be fully figured out.<br />
Since there is no known cure for the disease, symptoms<br />
are controlled through therapeutic interventions.<br />
Corticosteroids, mostly in the inhaled form, is the centerpiece<br />
of the current treatment to check the inflammatory process.<br />
Inhaled bronchodilators and/or leukotriene pathway inhibiting<br />
agents are also added if symptoms remain uncontrolled.<br />
The drug regimen, comprising inhaled corticosteroids<br />
(ICS) and long-acting beta agonists (LABA), has been used<br />
to manage asthma symptoms successfully in most of the<br />
patients.<br />
However, 10%–20% of patients do not achieve control<br />
with the current gold standard of care. This population of<br />
May 2019 / FUTURE MEDICINE / 17
slug<br />
Rapidly-expanding suite<br />
of biologics to reduce<br />
asthma exacerbations<br />
Monoclonal antibodies<br />
(mAbs) are antibodies<br />
produced artificially through<br />
genetic engineering and<br />
related techniques.<br />
mAbs target various<br />
proteins that influence cell<br />
activity, such as receptors or<br />
other proteins present on<br />
the surface of normal and<br />
cancer cells. These biologic<br />
therapeutics are currently<br />
used extensively<br />
to treat cancer,<br />
cardiovascular<br />
diseases,<br />
inflammatory conditions etc.<br />
Several mAbs have been developed<br />
to target asthma phenotypes.<br />
Omalizumab<br />
Omalizumab is directed against<br />
immunoglobulin E (IgE). It binds<br />
circulating IgE, causing decreased IgE<br />
levels, inhibition of IgE binding with its<br />
receptors, and downregulation of IgE<br />
receptors on mast cells, basophils and<br />
dendritic cells. This results in decreased<br />
release of inflammatory mediators<br />
related to the allergic response.<br />
Omalizumab is the only biologic<br />
therapy approved for paediatric use in<br />
children 6 years and older.<br />
A recent Cochrane review of<br />
omalizumab use in adults and children<br />
with asthma found that omalizumab use<br />
compared with placebo reduced asthma<br />
exacerbations.<br />
In the adolescent/adult studies, high<br />
levels of type 2 inflammatory markers<br />
such as fractional exhaled nitric<br />
oxide (FeNO) levels, eosinophilia,<br />
periostin levels, and IgE<br />
levels23 have predicted<br />
severe refractory asthmatics is at increased risk of morbidity<br />
and mortality, and make up the majority of the economic<br />
costs of asthma, studies indicate.<br />
Also, the long-term use of some asthma treatments,<br />
especially oral steroids, come with noticeable risks.<br />
Phenotypic pathways<br />
Search for newer, safer and better treatments for severe<br />
asthma has opened the door to exploring and identifying<br />
different types of this disease.<br />
Over the last decade, specific phenotypes and<br />
endotypes of asthma have been evaluated, leading to more<br />
personalised, targeted approaches to fit patient-specific<br />
disease, abandoning the one-size-fits-all treatments.<br />
A better understanding of the role of the inflammatory<br />
modulators involved in asthma paved the way for the<br />
development of therapies using biologics as a treatment<br />
option.<br />
Today, biologic therapies, which are made from<br />
living organisms using recombinant DNA technology, are<br />
increasingly being considered in patients with severe asthma.<br />
People with a severe form of the disease constantly struggle<br />
with persistent symptoms. This makes it tough for them to<br />
maintain a good quality of life.<br />
Traditionally, asthma is classified based on severity.<br />
Experts today hold that asthma is no longer single disease.<br />
Rather, it is increasingly recognised as an umbrella term — just<br />
like cancer or arthritis — for various phenotypes. Finding ways<br />
to identify subgroups that respond well to different types of<br />
treatments is a current, critical goal of asthma research.<br />
18 / FUTURE MEDICINE / May 2019
improved response to omalizumab.<br />
A 2003 analysis of pooled clinical<br />
trial data reported a malignancy risk of<br />
0.5% in omalizumab-treated patients.<br />
Ligelizumab<br />
Currently in development, ligelizumab<br />
is a humanized anti-IgE monoclonal<br />
antibody that binds circulating IgE. In<br />
a phase 1 study, ligelizumab has been<br />
shown to be more potent at suppressing<br />
free IgE and IgE bound to mast cells and<br />
basophils than omalizumab.<br />
In a phase 2 double-blind parallelgroup<br />
trial in 37 adults with mild allergic<br />
asthma, ligelizumab elicited a 3-fold<br />
greater provocative concentration of<br />
allergen, causing a 15% decrease in<br />
FEV1 compared with omalizumab and<br />
16-fold greater than placebo at 12<br />
weeks. Ligelizumab was administered<br />
subcutaneously every 2 weeks. So far,<br />
no studies have been done in children<br />
or adolescents, and there is no data on<br />
phenotypic response.<br />
Mepolizumab<br />
Mepolizumab targets IL5, a cytokine<br />
that promotes both the activation<br />
and longevity of eosinophils. The<br />
Mepolizumab as Adjunctive Therapy in<br />
Patients with Severe Asthma (MENSA)<br />
study randomised 576 adolescents and<br />
adults with severe eosinophilic asthma<br />
found that asthma exacerbations<br />
requiring oral corticosteroids (OCS) were<br />
reduced 47% and 53% with IV and SC<br />
mepolizumab compared with placebo,<br />
respectively. Asthma exacerbations<br />
requiring an ED visit were reduced 32%<br />
and 61% with intravenous (IV) and<br />
subcutaneous (SC) mepolizumab.<br />
The 3 randomized controlled trials<br />
included only patients with eosinophilic<br />
asthma on the basis that an antibody<br />
that reduces eosinophil levels would be<br />
most effective in eosinophilic asthma.<br />
Mepolizumab has been shown to<br />
decrease eosinophil counts in both<br />
serum and sputum.<br />
There are no studies on children<br />
above 12 years old, as of now, although<br />
many studies included adolescents.<br />
Mepolizumab is approved by USFDA<br />
as add-on maintenance therapy in<br />
patients who are 12 years and older<br />
with eosinophilic asthma.<br />
Reslizumab<br />
Two randomised, double-blind, placebocontrolled<br />
trials (RDBPCT) have been<br />
conducted on reslizumab in adolescents<br />
and adults with eosinophilic asthma.<br />
A phase 3 trial of 374 adolescents<br />
and adults aged 12–75 years with<br />
inadequately controlled eosinophilic<br />
asthma. This study noted improved<br />
asthma control compared with placebo.<br />
However, the quality of life measure<br />
trended to improve in the 0.3mg/<br />
kg dose of reslizumab but was only<br />
significant for the higher dose.<br />
Two duplicate RDBPCTs (phase<br />
3) in adolescents and adults with<br />
uncontrolled eosinophilic asthma with<br />
serum eosinophils =400 cells/µL on<br />
medium-to-high dose ICS therapy<br />
noted a significant reduction in asthma<br />
exacerbation frequency as well as<br />
improvements in time to exacerbation,<br />
asthma control, and quality of life<br />
As with mepolizumab, the presence<br />
of eosinophilia as a biomarker for<br />
reslizumab efficacy was demonstrated<br />
in an RDBPCT of reslizumab in adult<br />
asthmatics, which demonstrated no<br />
clinically significant effect on either lung<br />
function or symptom control in patients<br />
unselected for baseline eosinophil levels.<br />
Reslizumab has been shown to<br />
decrease blood eosinophil counts,<br />
but studies are not available to show<br />
differentiation between responders and<br />
One of these phenotypic pathways is thought to be type 2<br />
pathway — T helper 2 (Th2). About half of the individuals with<br />
severe asthma exhibit the type 2 phenotype, with increase in<br />
Th2 cells. There is an increase in the number of CD4+ T cells,<br />
predominantly TH2 cells, in the airways of asthmatic patients,<br />
whereas in normal airways, TH1 cells predominate. TH2 cells<br />
have a central role in asthmatic inflammation. Also called the<br />
“atopic” pathway, it refers to a hypersensitive reaction to an<br />
allergen. Patients with typ2 phenotype release cytokines such<br />
as interleukin [IL]-4, IL-5, IL-13, which drive immunoglobulin E<br />
(IgE) production.<br />
Targeting the cause<br />
The majority of asthma patients are also allergic. IgE, the<br />
antibody intimately involved in allergic responses, is the target<br />
of omalizumab, the first approved monoclonal antibody<br />
treatment for asthma.<br />
“Since omalizumab was first introduced to the United<br />
States market in 2003, it, and other more recent biologics<br />
for asthma, have made a tremendous difference in the lives<br />
of many asthmatics. Their impact can be likened to the<br />
introduction of the combination of inhaled steroids and longacting<br />
beta agonists in the 1990s, which allowed a number<br />
of asthmatics to attain greater control of their asthma and a<br />
number of severe asthmatics to get off of systemic steroids,”<br />
says Dr Andy Nish, MD Fellow of American Academy of<br />
Allergy, Asthma & Immunology (FAAAAI), USA.<br />
The asthma biologic drug research pipeline today contains<br />
12% monoclonal and 17% non-monoclonal antibodies.<br />
Some groups of white blood cells, such as eosinophils,<br />
May 2019 / FUTURE MEDICINE / 19
slug<br />
nonresponders in the magnitude of<br />
reduction in blood eosinophils.<br />
Reslizumab is USFDA approved for<br />
add-on therapy in adults aged 18 and<br />
older with eosinophilic asthma.<br />
Benralizumab<br />
An RDBPCT of 369 adults with severe<br />
asthma requiring oral steroid therapy<br />
use noted that benralizumab use of 30<br />
mg SC either every 4 weeks or every<br />
8 weeks reduced median final oral<br />
glucocorticoid dose from baseline by<br />
75%, compared with a 25% reduction<br />
in the placebo group. Benralizumab<br />
use reduced the annual exacerbation<br />
rate by 55% versus placebo when<br />
administered every 4 weeks and by 70%<br />
when administered every 8 weeks. No<br />
significant effect on forced respiratory<br />
volume 1 (FEV1) was noted.<br />
Increased blood eosinophil levels<br />
of more than 300 cells/µL have<br />
been identified in some studies as a<br />
biomarker of benralizumab efficacy.<br />
However, a pooled analysis of SIROCCO<br />
and CALIMA study data supported the<br />
use of benralizumab in patients with<br />
blood eosinophil counts =150 cells/<br />
µL73 and a newly released subsequent<br />
pooled analyses extended the efficacy<br />
irrespective of eosinophil count,<br />
although noting that the higher the<br />
eosinophil count the greater the benefit.<br />
Benralizumab is FDA approved<br />
for patients with severe asthma aged<br />
12 years and above, who have an<br />
eosinophilic phenotype.<br />
Dupilumab<br />
Dupilumab is a human monoclonal<br />
antibody to the alpha subunit of the IL4<br />
receptor, thereby blocking the activity of<br />
IL4 and IL13. Dupilumab is the only drug<br />
approved for self-administration.<br />
The IL4 cytokine is an essential<br />
cytokine to T helper 2 (Th2) cell<br />
polarization, whereas the IL13 cytokine<br />
is associated with periostin production<br />
in the bronchial epithelial cells,<br />
ultimately resulting in smooth muscle<br />
contraction, mucous production, airway<br />
remodeling and hyper-responsiveness.<br />
IL13 also works with IL4 to result in IgE<br />
production.<br />
An RDBPCT of dupilumab use in<br />
104 adults aged between 18 and 65<br />
years with moderate-to-severe<br />
asthma and high blood or sputum<br />
eosinophil counts above 300 cells/<br />
µL and 3% respectively on mediumto-high<br />
dose ICS plus LABAs resulted<br />
in an 87% reduction in asthma<br />
exacerbations and increased time to<br />
asthma exacerbation despite stopping<br />
LABA and ICS therapy while on<br />
dupilumab compared with placebo.<br />
There was noted improvement in<br />
FEV1 as early as the second week<br />
of treatment, which was maintained<br />
for the 12 weeks of the study. An<br />
RDBPCT of dupilumab using 300mg<br />
SC every 2 weeks for 24 weeks in 210<br />
adults with OCS-dependent asthma<br />
noted a 59% decrease in severe<br />
exacerbations in the dupilumab group<br />
despite a -70.1% reduction in OCS.<br />
An RDBPCT of 1902 adolescents<br />
and adults with uncontrolled asthma<br />
noted that dupilumab reduced severe<br />
exacerbations by 47.7% compared<br />
with placebo while increasing FEV1.<br />
Dupilumab has been shown to<br />
reduce FeNO levels and other levels<br />
of Th2 inflammation including TARC,<br />
eotaxin-3, and IgE.<br />
Dupilumab has been approved by<br />
US FDA for adolescents and adults aged<br />
12 years and older with moderate-to-<br />
are found to be critically involved in the inflammatory process<br />
in the airways that results in the loss of lung function in<br />
asthma. Several products have been developed to inhibit<br />
the production, the function, and the survival of eosinophils.<br />
These antibodies seem to work particularly well in individuals<br />
with high eosinophil counts prior to treatment and who<br />
have not responded to current treatment with inhaled<br />
corticosteroids. Collectively, these products are well tolerated<br />
and are effective in reducing eosinophil numbers and<br />
preventing asthma exacerbations.<br />
Multiple antibodies seek to tackle cytokines, which are<br />
key in fuelling allergic-type responses and inflammation. But<br />
the therapeutic agents that single out individual cytokines<br />
for targeting proved to be largely ineffective due to the<br />
overlapping effects of cellular signalling proteins. However,<br />
antibodies blocking the effect of more than one cytokine have<br />
shown promise.<br />
Mepolizumab, reslizumab, and benralizumab are targeted<br />
towards IL5, a cytokine that promotes both the activation and<br />
longevity of eosinophils. Dupilumab is an anti-IL4 cytokine<br />
antibody which is essential for Th2 cell polarization. Whereas,<br />
biologic tralokinumab, that targets IL13 cytokine, is currently<br />
under phase 2 development. IL13 is associated with periostin<br />
production in the bronchial epithelial cells, ultimately resulting<br />
in smooth muscle contraction, mucous production, airway<br />
remodelling and hyper-responsiveness.<br />
Today, clinicians have more than 10 years of clinical<br />
experience with IgE targeting omalizumab and extensive trial<br />
data with anti-eosinophil antibody mepolizumab. Unlike for<br />
most drugs, outcomes in clinics with omalizumab have often<br />
20 / FUTURE MEDICINE / May 2019
severe asthma as add-on maintenance<br />
therapy.<br />
Lebrikizumab<br />
Lebrikizumab is a humanized<br />
monoclonal antibody against IL13. IL13<br />
also works with IL4 to result in IgE<br />
production. The IL4 receptor (alpha<br />
subunit) is critical for both IL4 and IL13<br />
signal transduction.<br />
A phase 2 RDBPCT of lebrikizumab<br />
using 250 mg dosage strength SC once<br />
a month in 219 adults with asthma<br />
uncontrolled on medium-to-high<br />
dose ICS therapy noted a significant<br />
improvement in FEV1 after 12 weeks of<br />
lebrikizumab (5.5% points higher than<br />
placebo) although no significant change<br />
was noted in several other secondary<br />
efficacy outcomes including asthma<br />
control.<br />
Another phase 2 RDBPCT of 212<br />
adults aged 18–65 years not receiving<br />
ICS therapy noted no significant<br />
improvement in FEV1 with multiple<br />
different doses of lebrikizumab<br />
compared with placebo even with<br />
stratification based on periostin levels,<br />
although there was a reduced risk of<br />
treatment failure in all lebrikizumab dose<br />
groups.<br />
Periostin levels have emerged from<br />
the mentioned studies as a biomarker<br />
strongly correlated with lebrikizumab<br />
efficacy. Lebrikizumab has been<br />
associated with a decrease in FeNO,<br />
with greater reductions in FeNO in the<br />
high periostin groups.<br />
There are a small number of studies<br />
thus far that are limited to the adult<br />
population<br />
Tralokinumab<br />
Tralokinumab targets IL13. A phase<br />
2 RDBPCT of multiple doses of<br />
tralokinumab in 194 adults aged 18–65<br />
years with moderate-to-severe asthma<br />
noted no significant improvement in<br />
control with tralokinumab although<br />
there was a significant decrease in betaagonist<br />
use.<br />
A phase 2 RDBPCT of tralokinumab<br />
in 452 adults with severe uncontrolled<br />
asthma noted that tralokinumab use of<br />
300 mg SC every 2 or 4 weeks did not<br />
result in significant percentage change<br />
in annual asthma exacerbation rates.<br />
Prebronchodilator FEV1 was significantly<br />
increased in the tralokinumab every 2<br />
weeks group compared with placebo<br />
but not in the tralokinumab every<br />
4 weeks group. Subgroup analysis<br />
identified adults with a high baseline<br />
serum dipeptidyl peptidase-4, or high<br />
serum periostin levels had improved<br />
FEV1, asthma control and exacerbation<br />
rates.<br />
Tezepelumab<br />
Tezepelumab is a human anti-TSLP<br />
monoclonal immunoglobulin that<br />
prevents binding of TSLP with its<br />
receptor, preventing TSLP-initiated<br />
inflammatory responses through the<br />
activation of dendritic cells and mast<br />
cells. There has only been one proofof-concept<br />
RDBPCT of tezepelumab<br />
in 31 adults with allergic asthma that<br />
noted attenuation of both the early and<br />
late asthmatic responses, with a 45.9%<br />
smaller decrease in FEV1 during the<br />
late phase response after 12 weeks of<br />
treatment.<br />
A phase 2 RDBPCT of tezepelumab<br />
in adults with uncontrolled asthma<br />
despite medium-to-high ICS and<br />
LABA therapy noted significant<br />
reductions in exacerbation rates with<br />
tezepelumab, independent of baseline<br />
serum eosinophil levels, as well as an<br />
improvement in FEV1. No studies have<br />
been done in the paediatric population,<br />
the biomarker pro<strong>file</strong> of those most likely<br />
to respond remains unknown.<br />
Many small molecule drugs are also<br />
showing great promise.<br />
been better than those seen in clinical<br />
trials. It is considered a surprising feature of<br />
omalizumab in clinical practice.<br />
Rising incidence; dearth of biomarkers<br />
Estimates show that the incidence of<br />
asthma <strong>may</strong> have increased as much as<br />
12% over the past decade. Several theories<br />
postulate as to why the incidence of asthma<br />
is on the rise.<br />
First, asthma is more recognized and<br />
coded as such, as opposed to in the past,<br />
when it <strong>may</strong> have been diagnosed using<br />
terms such as bronchitis, explains Dr Nish.<br />
According to him, allergic diseases, in<br />
ESTIMATES SHOW<br />
THAT THE INCIDENCE<br />
OF ASTHMA MAY HAVE<br />
INCREASED AS MUCH<br />
AS 12% OVER THE PAST<br />
DECADE<br />
general, have been increasing; not only<br />
asthma, but also allergic rhinitis, food<br />
allergy and atopic dermatitis. “One theory<br />
is called the hygiene hypothesis, that<br />
our lymphocytes no longer have to fight<br />
infection as much as in the past, so they are<br />
becoming more Th2 cells and producing<br />
allergic disease,” he remarks.<br />
Air pollution has been linked to<br />
increased incidence of asthma in children,<br />
as has exposure to cigarette smoke,<br />
particularly if the mother smokes while<br />
pregnant. In addition, if a child’s diet is<br />
leading to obesity, it can be associated with<br />
an increased risk of asthma. Caesarean<br />
May 2019 / FUTURE MEDICINE / 21
irths change gut flora and increase the risk of asthma too.<br />
So, the rise in asthma is likely multifactorial.<br />
Even though biologic therapies allow the selection of<br />
patients suitable for intervention with antibody infusions,<br />
defining a set of predictive and monitoring biomarkers<br />
to assess the likelihood of a patient who will respond to<br />
a biologic continues to be a challenge. It is also difficult<br />
to determine whether there is a favourable response to<br />
continuing the biologic. Biomarkers, which will provide a<br />
pro<strong>file</strong> of those patients most likely to respond favourably and<br />
also to discontinue medication if there is a low likelihood of a<br />
response, are yet to be a reality. It took several years to define<br />
the subgroup of patients with severe asthma who respond to<br />
anti-IL-5 treatment.<br />
The concept of subtypes of asthma is new and clinically<br />
still evolving. Much has been made of endotypes, with many<br />
different pathways described as potentially contributing to<br />
the asthma phenotype. There has been much debate about<br />
type 2 (IgE and eosinophilia driven by IL-4, IL-5 and IL-13) and<br />
non-type 2 asthma. The RASP-UK study, adjusting therapy<br />
according to type 2 biomarkers, exhaled nitric oxide, blood<br />
eosinophils and serum periostin (an IL-13 induced epithelial<br />
“We recognize<br />
that asthma<br />
treatment is not<br />
‘one size fits all’”<br />
Andy Nish, MD is Fellow of American<br />
Academy of Allergy, Asthma &<br />
Immunology (FAAAAI) and the President<br />
of Northeast Georgia Physicians Group<br />
(NGPG) — Allergy and Asthma, GA. Dr Nish<br />
talks about the impact of biologics therapy<br />
in asthma, as well as the other advances<br />
in better defining and managing the<br />
chronic airway inflammatory disease. Edited<br />
excerpts:<br />
Nearly half a dozen biologics are now<br />
approved to treat asthma. And many more<br />
are under development. Can biologics<br />
eventually replace the current line of<br />
steroid-centred management of the<br />
symptoms and offer a lasting solution, if<br />
not a permanent cure, for the disease?<br />
Biologics have made a tremendous<br />
difference in the lives of many asthmatics.<br />
However, it is unlikely that the biologics<br />
will be able to completely replace current<br />
asthma therapies. For one, the biologics<br />
target a specific pathway which provides a<br />
narrower therapeutic efficacy than current<br />
standard therapies. And asthmatics in the<br />
intermittent, mild persistent and moderate<br />
persistent categories can typically be<br />
controlled with currently available therapies<br />
with a very acceptable risk to benefit<br />
ratio. In addition, the current costs of<br />
manufacturing the biologics make them<br />
cost-prohibitive to be used broadly.<br />
Unfortunately, the currently available<br />
biologics have not been shown to have<br />
disease-modifying properties for asthma.<br />
Studies have shown that asthma returns<br />
to its previous state of inflammation and<br />
pathology over time once the biologics are<br />
stopped.<br />
So, it’s unlikely that biologics, as we<br />
currently know them, can function as<br />
22 / FUTURE MEDICINE / May 2019
monotherapy for asthma or produce a<br />
cure, but then twenty years ago who<br />
could have known of the advances we<br />
have available today.<br />
Apart from monoclonals, what are<br />
the other approaches using biologics,<br />
currently being explored to address<br />
asthma?<br />
Despite the advances and benefits<br />
of currently available biologics, which<br />
are genetically engineered proteins,<br />
continued improvement is welcomed<br />
and there are definitely holes in our<br />
treatment choices. For the Th17 (non<br />
T2) type asthmatic, there are currently<br />
no highly effective biologics. Whereas<br />
currently 98% of all asthma treatment<br />
is small particle, the research pipeline<br />
contains 12% monoclonal and 17%<br />
non-monoclonal antibodies.<br />
The incidence of asthma is on<br />
the rise the world over despite<br />
wider availability of effective and<br />
comparatively less costly medicines<br />
to control the attacks. Why?<br />
It is estimated that asthma <strong>may</strong><br />
have increased as much as 12% over<br />
the past decade. There are a number<br />
of theories as to why the incidence<br />
of asthma is on the rise. In reality, it is<br />
probable that a number of these are<br />
contributing.<br />
First, it is suggested that asthma is<br />
more recognized and coded as such.<br />
Allergic disease, in general, has<br />
been increasing, not only asthma<br />
but also allergic rhinitis, food allergy,<br />
and atopic dermatitis. One theory is<br />
called the hygiene hypothesis, that our<br />
lymphocytes no longer have to fight<br />
infection as much as in the past, so<br />
they are becoming more Th2 cells and<br />
producing allergic disease.<br />
Air pollution has been linked to<br />
increased incidence of asthma in<br />
children, as has exposure to cigarette<br />
smoke, particularly if the mother<br />
smokes while pregnant. In addition, if<br />
a child’s diet is leading to obesity and<br />
overweight, it can be associated with<br />
an increased risk of asthma. Birth by<br />
It’s unlikely that biologics,<br />
as we currently know<br />
them, can function as<br />
monotherapy for asthma or<br />
produce a cure.<br />
Andy Nish MD<br />
Fellow of American Academy of<br />
Allergy, Asthma & Immunology, USA<br />
c-section changes the gut flora and<br />
increases the risk of asthma also.<br />
So, the rise in asthma is likely<br />
multifactorial, but the good news is<br />
that we do have more and better<br />
asthma medicines to use over time.<br />
Some of the experts in the field<br />
argue that the term “asthma”<br />
needs to be redefined, giving more<br />
emphasis on the heterogeneity of<br />
the disease. What is your comment?<br />
I definitely agree with this concept.<br />
As we have learned particularly<br />
in recent years, there is so much<br />
heterogeneity in the pathology of<br />
asthma and the inflammation thereof<br />
and response to treatment.<br />
The more medicines that have been<br />
developed, the more we learn about<br />
which patients do or don’t respond to<br />
particular of those medicines, and<br />
then research helps to delineate why<br />
that is.<br />
Some patients are primarily T2<br />
driven and some primarily Th17 driven.<br />
Some are primarily eosinophil driven<br />
and some primarily neutrophils. Some<br />
asthmatics respond dramatically to very<br />
small doses of inhaled corticosteroids<br />
and some respond minimally to<br />
very high doses of inhaled or even<br />
systemic steroids. It’s important that we<br />
recognize that asthma treatment is not<br />
“one size fits all”.<br />
Are corticosteroids being<br />
overprescribed for managing<br />
asthma?<br />
If the question is in regard to<br />
inhaled steroids, I would suggest<br />
that the answer is no. It <strong>may</strong> even be<br />
that inhaled steroids are not being<br />
prescribed often enough, partly<br />
because of steroid phobia, particularly<br />
on the part of parents. Other studies<br />
have suggested that asthma is<br />
underdiagnosed in general and the<br />
severity is underappreciated.<br />
It is recommended by experts<br />
that the drug of choice for the firstline<br />
treatment of mild, moderate<br />
and severe persistent asthma is<br />
inhaled steroids, of course with other<br />
medicines as needed as severity<br />
increases.<br />
We have good evidence from<br />
longitudinal studies that, if inhaled<br />
steroids affect children’s final adult<br />
height, it is a minimal effect and that<br />
other potential side effects, in general,<br />
present a favourable risk to benefit<br />
ratio. It is worth pointing out that<br />
inhaled steroids are in microgram<br />
doses and systemic steroids, if needed<br />
for an asthma flare, are in milligram<br />
doses, or 1000 times stronger.<br />
It is important to note that<br />
objective measures, such as<br />
pulmonary function tests, should be<br />
used to make sure that treatment is<br />
effective, and at the lowest possible<br />
dose for the fewest side effects.<br />
May 2019 / FUTURE MEDICINE / 23
protein), should help define which patients have controlled<br />
type 2 disease, and which have a non-type 2 mechanism<br />
driving their symptoms.<br />
The outlook for severe non-allergic asthma, however, is<br />
less rosy at present. This is because a lot of efforts have been<br />
devoted to finding effective drugs for severe allergic asthma,<br />
but severe non-allergic asthma has not been the focus of<br />
as much research. This is an area where more research is<br />
urgently needed, believes leading organisations like Asthma<br />
UK.<br />
But many researchers are still uncertain about the<br />
existence of non-type 2 asthma in the severe asthma<br />
population.<br />
“For the Th17 (non T2) type asthmatic, there are currently<br />
no highly effective biologics,” points out Dr Nish.<br />
Clinical trials should inform on the role of biomarkers<br />
in determining treatment response. Benefits of anti-<br />
IL-5 approaches with mepolizumab, reslizumab and<br />
benralizumab, and dupilumab (anti-IL4/IL-13) have been<br />
documented by large clinical trials. Phase III studies of<br />
lebrikizumab (anti-IL-13) did not show significant clinical<br />
benefit compared to placebo. The lebrikizumab phase 3<br />
studies did not enrich for exacerbations.<br />
Efficacy in children — Expanding evidence<br />
Asthma in childhood is quite different from asthma in<br />
adulthood. Phenotypically, paediatric asthma is more<br />
commonly allergic in nature. Likewise, the prevalence of<br />
asthma is higher in male children, whereas adult females<br />
have more severe and persistent disease. Hence, results<br />
from adult studies <strong>may</strong> not be extrapolated to the paediatric<br />
population. Evidence is expanding for biologic therapies<br />
such as omalizumab in children, despite the fact that<br />
evidence is limited for most of these drugs in the case of<br />
adult-onset asthma.<br />
Omalizumab is now approved up to 6 years of age in<br />
some countries. This makes it the first choice for children<br />
with severe asthma if the serum IgE is within limits.<br />
Mepolizumab and benralizumab, the anti-IL5 therapies, are<br />
available for use in some countries for adolescents of 12<br />
years of age. The treatment is likely to reduce exacerbations<br />
in cases where the blood eosinophil count is more than 300<br />
cells/µL and perhaps 150 cells/µL. Dupilimab, which has<br />
been found not having significant adverse effects in adult<br />
studies, is being considered for home administration.<br />
Adjunctives for uncontrolled asthma<br />
Omalizumab and anti-IL5 medications are currently<br />
included for use in patients with severe asthma, including<br />
children and adolescents, by several international asthma<br />
guidelines. The National Heart, Lung and Blood Institute’s<br />
Expert Panel 3 (NHLBI EPR3) guideline recommends the<br />
use of omalizumab as adjunctive therapy in patients above<br />
12 years who have atopic, severe and persistent asthma<br />
NOVEL BIOLOGIC AND SMALL MOLECULAR THERAPIES<br />
IN ASTHMA<br />
MEDICATION<br />
Omalizumab<br />
Ligelizumab<br />
Mepolizumab<br />
Reslizumab<br />
Benralizumab<br />
Dupilumab<br />
Lebrikizumab<br />
Tralokinumab<br />
Tezepelumab<br />
Fevipiprant<br />
MECHANISM OF<br />
ACTION<br />
Binds circulating<br />
IgE—↓IgE levels,<br />
downregulation of IgE<br />
receptors on mast cells,<br />
basophils, and dendritic<br />
cells.<br />
Binds circulating<br />
IgE—↓IgE levels,<br />
downregulation of IgE<br />
receptors on mast cells,<br />
basophils, dendritic cells.<br />
Binds IL5, which recruits<br />
eosinophils from the<br />
bone marrow and<br />
promotes activation and<br />
longevity of eosinophils<br />
Binds IL5, which recruits<br />
eosinophils from the<br />
bone marrow and<br />
promotes activation and<br />
longevity of eosinophils<br />
Binds IL5 receptor,<br />
preventing binding of<br />
IL5<br />
Blocks activity of IL4<br />
and IL13<br />
Binds IL13, thereby<br />
blocking its activity<br />
Binds IL13, thereby<br />
blocking its activity<br />
Prevents binding of<br />
TSLP with its receptor,<br />
preventing TSLPinitiated<br />
inflammatory<br />
response through<br />
activation of dendritic<br />
cells and mast cells<br />
Antagonist to CRTh2,<br />
which is a PGD2<br />
receptor that mediates<br />
inflammatory effects<br />
through production of<br />
mast cells and other<br />
allergic cells<br />
MODE OF<br />
ADMINISTRATION<br />
SC every 2–4<br />
weeks based on<br />
body weight and<br />
serum IgE level<br />
SC every 2 weeks<br />
SC every 4 weeks<br />
IV every 4 weeks<br />
SC every 4 or 8<br />
weeks<br />
SC every 2 week<br />
SC every 4 weeks<br />
SC every 2–4<br />
weeks<br />
IV every 2–4 weeks<br />
Orally once a day<br />
inadequately controlled with high-dose ICS and LABA<br />
therapy.<br />
The Global Initiative for Asthma (GINA) 2017 update<br />
recommends phenotype-guided add-on treatment with<br />
omalizumab in patients above 6 years with severe allergic<br />
asthma with elevated IgE, and mepolizumab or reslizumab<br />
in patients above 12 years with eosinophilic asthma as step<br />
24 / FUTURE MEDICINE / May 2019
CALCILYTICS OFFER A<br />
NEW LINE OF ASTHMA<br />
THERAPY<br />
Researchers at the MRC-Asthma UK<br />
Centre in Allergic Mechanisms of<br />
Asthma and Cardiff University found<br />
that people with asthma have more of a<br />
special type of calcium receptor in their<br />
lungs than people without asthma.<br />
Called calcium-sensing receptors or<br />
CaSR, they sit on the surface of a cell,<br />
respond to chemicals in the environment<br />
such as pollutants or allergens that trigger<br />
asthma.<br />
The scientists later found that a class<br />
of drugs called calcilytics can inhibit<br />
CaSRs.<br />
‘Calcilytic’ drugs can reverse or abolish<br />
twitchiness of the airways which are<br />
responsible for the symptoms of asthma,<br />
say researchers.<br />
Existing drugs, such as preventer<br />
inhalers, address this problem only<br />
indirectly, by reducing the inflammation<br />
of the airways which is thought to be one<br />
of the triggers for this twitchiness, while<br />
reliever inhalers make the muscle cells<br />
relax, temporarily alleviating the blockage.<br />
By using calcilytic drugs, the cause of<br />
the twitchiness can be directly targeted,<br />
instead of working on the reaction,<br />
regardless of what has caused it. It can<br />
thus treat all asthma.<br />
The challenge now is to develop a<br />
form of these drugs which can be inhaled<br />
safely to the airways and then explore<br />
their effects in people with asthma.<br />
The next logical step will be clinical<br />
trials to see if the drugs work.<br />
4 (medium dose ICS +LABA) therapy.<br />
The Canadian Thoracic Society (CTS) recommends<br />
omalizumab in patients above 6 years with severe asthma<br />
that is inadequately controlled on high dose ICS and at least<br />
1 other controller, sensitization to more than 1 perennial<br />
aeroallergen, and serum IgE levels between 30 and 1,300<br />
IU/mL (6–11 years) or 30–700 IU/mL in patients above 12<br />
May 2019 / FUTURE MEDICINE / 25
years. The CTS also recommends anti-IL5 therapies in adults<br />
with severe eosinophilic corticosteroid-dependent asthma<br />
in an attempt to decrease or withdraw oral corticosteroids<br />
(OCS).<br />
Benefits ‘at best Incremental’<br />
Omalizumab, mepolizumab, reslizumab, benralizumab,<br />
and dupilumab are currently the five US FDA-approved<br />
monoclonal antibodies that affect the pathways involved<br />
in either allergic or type 2 inflammatory phenotypes of<br />
asthma. Presently, there are no head to head randomized<br />
or observational trials to study the comparative clinical<br />
effectiveness of these mAbs. Estimates from Cochrane<br />
meta-analyses showed that all five drugs reduced the<br />
annual exacerbation rate by about 50% with overlapping<br />
confidence intervals. The average improvement for four of<br />
the drugs, compared with a placebo, is modest and none<br />
of them reach the minimally important difference, although<br />
all were statistically significant. Dupilumab had the largest<br />
reduction in exacerbations and benralizumab the smallest,<br />
according to a meta-analysis by the Institute for Clinical<br />
and Economic Review (ICER), an independent nonprofit<br />
research organisation. The risk for serious<br />
adverse events was low for all five drugs. The only<br />
consistent and common adverse event was<br />
injection-site reactions. The dosing schedule<br />
varies between the drugs. Dupilumab is<br />
given every two weeks, omalizumab is given<br />
every two to four weeks, mepolizumab and<br />
reslizumab are given every four weeks, and after the first<br />
three doses, benralizumab is given every eight weeks.<br />
Notably, none of the drugs prevented most<br />
exacerbations requiring systemic corticosteroids or improved<br />
average daily quality of life to a degree considered clinically<br />
significant. Thus, the overall health benefit for all five drugs<br />
is at best incremental, notes ICER report.<br />
“Unfortunately, the currently available biologics have<br />
not been shown to have disease-modifying properties for<br />
asthma,” comments Dr Nish, who is also the President of<br />
Northeast Georgia Physicians Group (NGPG) — Allergy and<br />
Asthma, GA. Studies have shown that asthma returns to<br />
its previous state of inflammation and pathology over time<br />
once the biologics are stopped, he adds.<br />
The long-term safety and effectiveness of these<br />
drugs, however, is yet to be established particularly in<br />
older patients. For omalizumab and mepolizumab, longterm<br />
extension trials and real-world experience provide<br />
supportive but uncontrolled evidence. Response to therapy<br />
is yet to be well-defined to help guide patients and<br />
clinicians in deciding when to stop one therapy and consider<br />
switching to another.<br />
At what cost?<br />
Biologics comes with high treatment costs. Analysis indicate<br />
that biologic agents provide gains in quality-adjusted<br />
survival over the standard of care alone. Exacerbation<br />
reductions and chronic oral steroid reductions are<br />
potentially the most influential benefit associated with<br />
biologic therapy.<br />
A survey by the Asthma and Allergy Foundation of<br />
America involving 805 Americans living with asthma,<br />
including 185 with severe, uncontrolled asthma, found that<br />
an average of 82% chose effectiveness as a key criterion<br />
while an average of 52% cited cost as a key criterion for<br />
selecting a therapy.<br />
Patients report that their asthma prevents them from<br />
living the life that they want to live, besides impacting their<br />
loved ones. They also fear the side effects of corticosteroids<br />
and want to minimize the use of both systemic and inhaled<br />
corticosteroids as much as possible.<br />
26 / FUTURE MEDICINE / May 2019
Looking for loci<br />
Asthma is a multifactorial<br />
disease with a complex genetic<br />
inheritance. Collective evidence<br />
suggests that genetic factors account<br />
for approximately 60–80% of its<br />
susceptibility. More than a hundred<br />
genetic variations positioned<br />
throughout the genome have been<br />
implicated in asthma susceptibility.<br />
However, studies could replicate only<br />
a subset of these genes. Besides, the<br />
exact mechanism of the interaction of<br />
these genotypes with the environment<br />
is not clearly understood.<br />
The incidence of asthma, which<br />
is the highest in childhood, tends to<br />
show a bias towards boys. While boys<br />
are at a higher risk of asthma in early<br />
childhood, girls are more frequently<br />
affected after puberty. Hypersensitivity<br />
to aeroallergens is the key feature<br />
of childhood-onset asthma. Atopic<br />
dermatitis and hay fever are closely<br />
associated with asthma, constituting<br />
what is known as the atopic triad.<br />
Genetic factors determining the<br />
age of onset of asthma has been<br />
traced to diverse chromosomal loci.<br />
Two regions — 5q13 and 1p31 — with a<br />
suggestive linkage to time of onset of<br />
asthma, have been specifically identified<br />
in French families. Also, a region on<br />
7q was found linked to asthma in the<br />
same population, but with different<br />
risks. The -28G allele of the RANTES<br />
promoter region at chromosome 17q<br />
increased the risk of late-onset asthma<br />
in Japanese individuals<br />
A genome-wide association (GWA)<br />
study revealed that the 17q12-21 region<br />
(IKZF3-ZPBP2-GSDMB-ORMDL3 region)<br />
IN SPITE OF THE HIGH<br />
HERITABILITY OF ASTHMA<br />
SUSCEPTIBILITY, GENETIC<br />
FACTORS ACCOUNT FOR<br />
ONLY AROUND 35%<br />
OF THE VARIATION IN<br />
THE AGE AT ONSET<br />
is predominantly a locus of childhoodonset<br />
asthma. Despite these findings,<br />
it is not clear whether sex differences<br />
in asthma risk at different ages can be<br />
explained by genetic factors.<br />
Molecular genetic studies suggest<br />
an association of a variant on<br />
chromosome 5, situated within the<br />
TSLP gene, among patients with severe<br />
asthma. Similarly, polymorphisms within<br />
the beta2-adrenergic receptor gene,<br />
the IL-4 gene and the TGF-ß1 and CD14<br />
genes have been shown to play a role<br />
in asthma severity.<br />
Positional cloning identified five<br />
asthma genes or gene complexes,<br />
including ADAM33, PHF11, DPP10, GRPA<br />
and SPlNK5. Though the functions of<br />
these genes are largely obscure, the<br />
expression of DPP10, GRPA and SPlNK5<br />
in terminally differentiating epithelium<br />
suggests that they respond to the<br />
external environment.<br />
Many of the genes identified by<br />
candidate gene studies, including lL13<br />
that modifies mucous production, FccRl-<br />
/J which modifies an allergic trigger<br />
on mast cells, and microbial pattern<br />
recognition receptors of the innate<br />
immune system, <strong>may</strong> also exert their<br />
effects within the cells that make up the<br />
mucosa.<br />
There is also evidence of genetic<br />
heterogeneity within the clinical<br />
expression of asthma. In spite of the<br />
high heritability of asthma susceptibility,<br />
genetic factors account for only around<br />
35% of the variation in the age at onset<br />
and for around 25% of the variation<br />
in the overall symptomatic severity of<br />
the disease and for even less of the<br />
variation in the severity of individual<br />
asthma symptoms such as wheezing,<br />
shortness of breath, chest tightness and<br />
cough, according to studies.<br />
Given the high cost of these therapies, GINA<br />
recommends the use of biologics in patients whose<br />
asthma is refractory or relatively refractory to<br />
conventional inhaled therapies.<br />
Biologics are also indicated for eosinophilic<br />
asthma. Eosinophils are part of the immune<br />
response to parasitic infections. It is unknown if the<br />
therapies that decrease eosinophil counts will affect<br />
patients’ ability to fight such infections. Current<br />
guidelines recommend that physicians treat patients for<br />
existing parasitic infections prior to initiating anti IL-5<br />
therapy.<br />
Researchers call for designing a large, pragmatic trial<br />
comparing all available drugs in order to clarify whether<br />
or not there are clinically important differences between<br />
the drugs.<br />
May 2019 / FUTURE MEDICINE / 27
ASTHMA ALERT<br />
Asthma is increasing in many parts of the world,<br />
notably in developing nations.<br />
339<br />
million<br />
people suffer from asthma. It is<br />
estimated that asthma has increased as<br />
much as 12% over the past decade<br />
383,000<br />
people die of asthma every year<br />
INFOGRAPHICS: GOPAKUMAR K<br />
SOURCE: WHO/ GLOBALASTHMAREPORT.ORG<br />
80%<br />
deaths occur in<br />
low and<br />
lower-middle<br />
income<br />
countries<br />
28 / FUTURE MEDICINE / May 2019
WORLD vs INDIA<br />
A CHANGING CONCEPT?<br />
14%<br />
Children<br />
6%<br />
22.6%<br />
8%<br />
INHALER MARKET<br />
Americans use more inhalers<br />
than any other population<br />
8%<br />
15%<br />
45%<br />
Asia Pacific<br />
Europe<br />
of population<br />
suffer from<br />
asthma<br />
globally<br />
of Indian<br />
population are<br />
asthmatics<br />
32%<br />
8.6%<br />
Adult<br />
2%<br />
Middle East & Africa<br />
America<br />
Asthma is a well-recognized medical condition.<br />
However, there is not one universal, agreed-upon<br />
definition for the disease<br />
The Global Initiative for Asthma (GINA)<br />
defines the condition as “a chronic<br />
inflammatory disorder of the airways in<br />
which many cells and cellular elements<br />
play a role. The chronic inflammation<br />
is associated with airway hyperresponsiveness<br />
that leads to recurrent<br />
episodes of wheezing, breathlessness,<br />
chest tightness and coughing, particularly<br />
at night or in the early morning. These<br />
episodes are usually associated with<br />
widespread but variable airflow obstruction<br />
within the lung that is often reversible<br />
either spontaneously or with treatment”.<br />
Concepts of asthma have changed<br />
substantially over the past 50 years.<br />
GINA now explicitly emphasises the<br />
heterogeneity of asthma and uses the<br />
term asthma as an umbrella term like<br />
anaemia, arthritis and breast cancer.<br />
Unlike with anaemia, arthritis or cancer,<br />
evidence about underlying mechanisms of<br />
asthma is incompletely understood.<br />
Years ago, when we had fewer<br />
medicines with which to treat asthma,<br />
and a much more basic concept of the<br />
disease as primarily bronchoconstriction,<br />
it was easier to think of it as one<br />
disease, says Dr Andy Nish, MD, Fellow of<br />
American Academy of Allergy, Asthma &<br />
Immunology (FAAAAI), USA.<br />
“Now, it’s probably much more<br />
appropriate to think of it as a syndrome<br />
whose patients have in common<br />
reversible airflow disease, bronchial<br />
hyperresponsiveness, airway inflammation<br />
and typically symptoms of wheezing,<br />
coughing, shortness of breath and chest<br />
tightness brought on by some common<br />
factors such as viruses, weather changes,<br />
exercise, smoke,etc, “ he adds.<br />
Many studies exploring the<br />
inflammatory pathways are restricted to<br />
patients with severe asthma.<br />
For the diagnosis and management<br />
of asthma, clinicians rely on evidencebased<br />
recommendations, including those<br />
developed as formal clinical practice<br />
guidelines. Different bodies make different<br />
recommendations from the same<br />
evidence, as is the case with the UK in<br />
asthma guidelines published by the British<br />
Thoracic Society/Scottish Intercollegiate<br />
Guideline Network (BTS/SIGN) and the<br />
National Institute for Health and Care<br />
Excellence (NICE).<br />
Therefore, it is essential to have<br />
national or regional guidelines in order to<br />
provide locally-specific recommendations.<br />
Spirometry and breathomics<br />
Presently, there is no precise test for<br />
asthma diagnosis. Diagnosis is typically<br />
based on the pattern of symptoms<br />
and the response to therapy over time.<br />
Clinicians suspect asthma if there is a<br />
history of recurrent wheezing, coughing<br />
or difficulty in breathing, and these<br />
symptoms occur or worsen due to<br />
exercise, viral infections, allergens or air<br />
pollution. Spirometry is then used to<br />
confirm the diagnosis.<br />
Spirometry is the single best test<br />
for asthma. If the FEV1 measured by<br />
this technique improves more than<br />
12% and increases by at least 200<br />
milliliters following the administration of<br />
a bronchodilator such as salbutamol, it is<br />
supportive of the diagnosis. It, however,<br />
<strong>may</strong> be normal in those with a history of<br />
mild asthma.<br />
COPD overlap<br />
Asthma is a chronic obstructive condition.<br />
But it is not considered as a part of<br />
chronic obstructive pulmonary disease<br />
(COPD). One of the most characteristic<br />
features of efficacy studies in asthma and<br />
COPD is their restriction to “pure” asthma<br />
and “pure” COPD, point out asthma<br />
researchers.<br />
COPD refers specifically to<br />
combinations of disease that are<br />
irreversible, such as bronchiectasis, chronic<br />
bronchitis and emphysema. Unlike these<br />
diseases, the airway obstruction in asthma<br />
is usually reversible; however, if left<br />
untreated, the chronic inflammation from<br />
asthma can lead the lungs to become<br />
irreversibly obstructed due to airway<br />
remodelling. Unlike emphysema, asthma<br />
affects the bronchi, not the alveoli.<br />
May 2019 / FUTURE MEDICINE / 29
pulmonology<br />
BRONCHIAL THERMOPLASTY<br />
DEVICE THERAPY FOR<br />
ASTHMA<br />
A novel modality, BT aims to prevent the structural changes in<br />
airway smooth muscle<br />
DR GEORGE MOTHI JUSTIN<br />
Asthma is a complex inflammatory<br />
disorder of the airways<br />
characterized by airway hyperresponsiveness<br />
(AHR) and variable<br />
airflow obstruction. Although advances<br />
in clinical and basic research over<br />
the past few decades have led to the<br />
development of effective treatments<br />
and dissemination of detailed disease<br />
management guidelines, difficult-totreat<br />
asthma continues to affect 5-10%<br />
of adults with this disorder.<br />
Bronchial thermoplasty (BT) is<br />
a modality for treating asthma and<br />
is thought to prevent the chronic<br />
structural changes that occur in airway<br />
smooth muscle (ASM) in individuals<br />
with asthma. BT targets ASM via the<br />
delivery of a controlled specific amount<br />
of radiofrequency (RF) energy (RF<br />
ablation [RFA]) to the airway wall<br />
through a dedicated catheter.<br />
It is an innovative treatment<br />
whose clinical efficacy and safety are<br />
beginning to be better understood.<br />
Since this is a device-based therapy,<br />
the overall evaluation of risk-benefit<br />
is unlike that of pharmaceutical<br />
products; safety aspects, regulatory<br />
requirements, study design and effect<br />
30 / FUTURE MEDICINE / May 2019
size assessment <strong>may</strong> be unfamiliar.<br />
The mechanisms of action and optimal<br />
patient selection need to be addressed<br />
in further rigorous clinical and scientific<br />
studies.<br />
Indications<br />
Candidates for bronchial thermoplasty<br />
include adults with severe persistent<br />
asthma who require regular<br />
maintenance medications of inhaled<br />
corticosteroids (>1000 µg/day of<br />
beclomethasone or the equivalent) and<br />
a long-acting beta agonist (≥100 µg/<br />
day of salmeterol or the equivalent).<br />
These patients would have received<br />
add-on therapies such as leukotriene<br />
modifiers, omalizumab, or oral<br />
corticosteroids (≤10 mg/day).<br />
These patients should be on stable<br />
maintenance asthma medications<br />
according to accepted guidelines,<br />
should have a pre-bronchodilator<br />
forced expiratory volume in 1 second<br />
(FEV1) of 60% or more of predicted,<br />
and should have a stable asthma<br />
status (FEV1 within 10% of the best<br />
value, no current respiratory tract<br />
infection, and no severe asthma<br />
exacerbation within the preceding 4<br />
weeks).<br />
Patients are usually selected<br />
on the basis of the AIR 2 trial. The<br />
patient should be stable in terms<br />
of asthma status, defined as a post<br />
bronchodilator FEV1 within 15% of<br />
baseline values with no respiratory<br />
tract infection or asthma exacerbations<br />
within the preceding 14 days.<br />
Contraindications<br />
Contraindications for BT include the<br />
following:<br />
• Presence of an implantable<br />
electronic device<br />
• Known hypersensitivity to drugs<br />
used during bronchoscopy<br />
• Severe comorbid conditions that<br />
would increase the risk of adverse<br />
events<br />
Patients are not considered<br />
candidates for BT if they had three or<br />
more hospitalisations for asthma, three<br />
or lower respiratory tract infections and<br />
four or more oral corticosteroids used<br />
for asthma in the previous year.<br />
Although the benefits of BT <strong>may</strong><br />
be large, the potential harm <strong>may</strong> be<br />
large as well, and the long-term side<br />
effects are unknown. Studies are still<br />
needed to assess exacerbation rates<br />
and long-term effects on lung function.<br />
It remains to be determined which<br />
phenotypes will respond best to BT,<br />
what the effects <strong>may</strong> be on obstructed<br />
patients with an FEV1 higher than<br />
60%, and what the applicability of the<br />
procedure <strong>may</strong> be in patients receiving<br />
systemic steroid therapy<br />
Procedure considerations<br />
BT is performed via fibreoptic<br />
bronchoscopy in three separate<br />
procedures, separated by<br />
approximately 3 weeks, as<br />
PATIENTS ARE NOT<br />
CONSIDERED CANDIDATES<br />
FOR BT IF THEY HAD THREE<br />
OR MORE HOSPITALISATIONS<br />
FOR ASTHMA<br />
demonstrated by previous studies.]<br />
Dividing the treatments into three<br />
bronchoscopy sessions minimizes<br />
the risk of inducing an asthma<br />
exacerbation or diffuse airway oedema.<br />
It also avoids excessive procedural<br />
length. BT takes longer (30-60<br />
minutes) than a standard fiber-optic<br />
bronchoscopy (5-20 minutes) does,<br />
and the longer duration implies the<br />
use of larger doses of medication for<br />
sedation.<br />
All accessible airways are treated,<br />
with the exception of the right middle<br />
lobe, because of the theoretical<br />
concern about the risk of inducing<br />
right-middle-lobe syndrome.]<br />
Oxygen delivery should be started<br />
via a nasal or oral cannula during<br />
the procedure, with appropriate<br />
monitoring of vital signs. Heart rate,<br />
pulse oximetry, and noninvasive blood<br />
pressure should be continuously<br />
monitored.<br />
Outcomes<br />
Patients <strong>may</strong> experience respiratoryrelated<br />
adverse events such as cough,<br />
wheezing, and chest tightness during<br />
the treatment period. Most of these<br />
symptoms occur within 1 day of the<br />
procedure and resolve in an average of<br />
7 days with standard therapy.<br />
It is unclear why an intervention<br />
aiming at a reduction of smoothmuscle<br />
mass would not affect<br />
FEV1. Given that the number of<br />
exacerbations is reduced with no<br />
change in FEV1, it <strong>may</strong> be that altered<br />
response to the inflammatory triggers<br />
plays a role in addition to the reduction<br />
in smooth-muscle mass.<br />
Complications<br />
Recurrent lung atelectasis secondary<br />
to fibrin plugs has been reported<br />
as an early complication of BT.<br />
In the susceptible patient, high<br />
thermal stimulation <strong>may</strong> lead to<br />
an inflammatory reaction with<br />
microvascular alteration, induced<br />
either by heat or by the release of<br />
inflammatory mediators. Lung abscess<br />
has also been described as a direct<br />
complication; thus, collecting and<br />
publishing safety data continue to<br />
be important. A prospective cohort<br />
study performed as part of the<br />
TASMA trial reported a high incidence<br />
of acute radiologic abnormalities<br />
after BT. Postprocedural CT of<br />
the chest identified four different<br />
radiologic patterns: (1) peribronchial<br />
consolidations with surrounding<br />
ground-glass opacities (94%), (2)<br />
atelectasis (38%), (3) partial bronchial<br />
occlusions (63%), and (4) bronchial<br />
dilatations (19%). These complications<br />
resolved without clinical impact in<br />
virtually all cases.<br />
The author is Head, Department of<br />
Respiratory Medicine, Medical Trust<br />
Hospitals, Cochin<br />
May 2019 / FUTURE MEDICINE / 31
drug approvals<br />
Hydrogel<br />
capsule for<br />
weight loss<br />
Plenity manufactured by the<br />
Gelesis has been granted<br />
approval by USFDA as an aid<br />
in weight management in<br />
adults with a body mass index<br />
(BMI) of 25–40 kg/m2, when<br />
used in conjunction with diet<br />
and exercise.<br />
It is an oral, non-systemic,<br />
superabsorbent hydrogel<br />
capsule which when taken<br />
together with diet and<br />
exercises aids in weight<br />
loss by inducing satiety and<br />
reducing hunger.<br />
The drug can be<br />
administered in the form<br />
of capsules that could be<br />
taken with water before<br />
dinner and lunch. It is made<br />
of two naturally derived<br />
compounds called cellulose<br />
and citric acid which are cross<br />
linked together in a manner<br />
that they create a threedimensional<br />
matrix.<br />
The hydrogel-based<br />
capsule release thousands<br />
of non-aggregating gel-like<br />
particles that rapidly<br />
absorbs water in the stomach.<br />
These small gellike particles<br />
have an elasticity of plantbased<br />
foods but they do<br />
not have any caloric value.<br />
These gel particles increase<br />
the elasticity of the contents<br />
stored in the stomach and<br />
intestine giving out a feeling<br />
of fullness that aids in weight<br />
loss.<br />
The clinical studies<br />
of Plenity have shown<br />
positive effects on weight<br />
management.<br />
Breakthrough<br />
therapy<br />
designation to<br />
elafibranor<br />
Genfit announced that its<br />
lead product candidate<br />
elafibranor was granted<br />
Breakthrough Therapy<br />
Designation by the US FDA<br />
for the treatment of primary<br />
biliary cholangitis (PBC)<br />
in adults with inadequate<br />
response to ursodeoxycholic<br />
acid (UDCA).<br />
Elafibranor is a first-inclass<br />
double peroxisome<br />
proliferator-activated receptor<br />
alpha and delta (PPAR alpha/<br />
delta) agonist which has<br />
produced positive results<br />
in a phase 2 clinical trial<br />
evaluating its safety and<br />
efficacy in adults with PBC<br />
and inadequate response to<br />
UDCA.<br />
Elafibranor is also<br />
currently evaluated in a phase<br />
3 clinical trial in non-alcoholic<br />
steatohepatitis (NASH).<br />
Genfit presented detailed<br />
results from its positive phase<br />
2 clinical trial evaluating<br />
elafibranor in PBC during<br />
the European Association for<br />
Hormone therapy<br />
for menopausal<br />
symptoms<br />
The US FDA has approved<br />
first of its kind capsule<br />
Bijuva which contains<br />
progesterone and<br />
estradiol, announced<br />
TherapeuticMD.<br />
It is the first bioidentical<br />
hormone therapy<br />
combination that has been<br />
approved by the FDA.<br />
The approval is based<br />
on the Bijuva clinical<br />
development programme<br />
that included the pivotal<br />
phase III Replenish Trial.<br />
This trial evaluated the<br />
safety and efficacy of<br />
Bijuva in generally healthy,<br />
postmenopausal women<br />
with a uterus for the<br />
the Study of the Liver (EASL)<br />
annual International Liver<br />
Congress (ILC).<br />
In a 12-week doubleblind<br />
randomized placebocontrolled<br />
phase 2 trial of<br />
non-cirrhotic patients with<br />
PBC and with inadequate<br />
response to UDCA, elafibranor<br />
showed a significant decrease<br />
of alkaline phosphatase (ALP)<br />
levels, resulting in significant<br />
treatment of moderate to<br />
severe hot flashes.<br />
The co-primary efficacy<br />
endpoints in the Replenish<br />
Trial were the change from<br />
baseline in the number<br />
and severity of hot flashes<br />
at weeks 4 and 12 as<br />
compared to placebo.<br />
Bijuva demonstrated<br />
a statistically significant<br />
reduction from baseline<br />
in both the frequency and<br />
severity of hot flashes<br />
compared to placebo while<br />
reducing the risks to the<br />
endometrium. The results<br />
of the trial were published<br />
in the journal Obstetrics &<br />
Gynecology.<br />
treatment effects versus<br />
placebo on the primary<br />
endpoint.<br />
Gene-edited<br />
stem cell<br />
therapy for<br />
thalassemia<br />
T<br />
he US FDA has granted<br />
Fast Track Designation for<br />
CTX001 for the treatment of<br />
transfusion-dependent beta<br />
thalassemia (TDT).<br />
CTX001 is an<br />
investigational, autologous,<br />
gene-edited haematopoietic<br />
stem cell therapy for patients<br />
suffering from severe<br />
haemoglobinopathies.<br />
32 / FUTURE MEDICINE / May 2019
form of haemoglobin.<br />
The elevation of HbF by<br />
CTX001 has the potential<br />
to alleviate transfusion<br />
requirements for TDT patients<br />
and painful and debilitating<br />
sickle crises for SCD patients.<br />
Speedy approval<br />
for erdafitinib<br />
to treat bladder<br />
cancer<br />
had previously not responded<br />
to anti-PD-L1/PD-1 therapy.<br />
Erdafitinib is the first<br />
approved drug in a class<br />
known as FGFR inhibitors<br />
that targets growth factor<br />
receptors involved in cell<br />
growth and division.<br />
In February 2019, CRISPR<br />
Therapeutics and Vertex<br />
announced that the first<br />
patient had been treated with<br />
CTX001 in a phase 1/2 clinical<br />
study of patients with TDT,<br />
marking the first companysponsored<br />
use of a CRISPR/<br />
Cas9 therapy in a clinical trial.<br />
The Phase 1/2 open-label<br />
trial is designed to assess<br />
the safety and efficacy of<br />
a single dose of CTX001 in<br />
patients ages 18 to 35 with<br />
TDT, non-beta zero/beta zero<br />
subtypes. The companies are<br />
also evaluating CTX001 for the<br />
treatment of sickle cell disease<br />
(SCD) and received Fast Track<br />
Designation for CTX001 from<br />
the FDA in January 2019 for<br />
SCD.<br />
In CTX001 therapy, a<br />
patient’s haematopoietic<br />
stem cells are engineered<br />
to produce high levels of<br />
foetal haemoglobin (HbF)<br />
in red blood cells. HbF is a<br />
form of the oxygen-carrying<br />
haemoglobin that is naturally<br />
present at birth and is<br />
then replaced by the adult<br />
Erdafitinib (Balversa) has<br />
been granted accelerated<br />
approval by US FDA to treat<br />
adult patients with locally<br />
advanced or metastatic<br />
bladder cancer that has a<br />
type of susceptible genetic<br />
alteration known as FGFR3 or<br />
FGFR2.<br />
Patients should be<br />
selected for therapy with<br />
erdafitinib using an FDAapproved<br />
companion<br />
diagnostic device.<br />
The efficacy of erdafitinib<br />
was studied in a clinical trial<br />
that included 87 patients with<br />
locally advanced or metastatic<br />
bladder cancer, with FGFR3<br />
or FGFR2 genetic alterations,<br />
that had progressed following<br />
treatment with chemotherapy.<br />
The overall response rate<br />
in these patients was 32.2%,<br />
with 2.3% having a complete<br />
response and almost 30%<br />
having a partial response.<br />
The response lasted for an<br />
average of approximately fiveand-a-half<br />
months.<br />
Responses to erdafitinib<br />
were seen in patients who<br />
Sakigake desig<br />
for valemetostat<br />
in Japan<br />
D<br />
aiichi Sankyo announced<br />
that valemetostat, an<br />
investigational and potential<br />
first-in-class EZH1/2 dual<br />
inhibitor, has received<br />
Sakigake Designation for the<br />
treatment of adult patients<br />
with relapsed/refractory<br />
peripheral T-cell lymphoma<br />
(PTCL) by the Japan Ministry<br />
of Health, Labour and Welfare<br />
(MHLW).<br />
Valemetostat targets<br />
epigenetic regulation by<br />
inhibiting both the EZH1<br />
(enhancer of zeste homolog<br />
1) and EZH2 enzymes that act<br />
through histone methylation<br />
to regulate gene expression.<br />
Reactivation of the silenced<br />
genes results in decreased<br />
proliferation of EZH2-<br />
expressing cancer cells.<br />
Preclinical research has<br />
shown that valemetostat<br />
suppressed trimethylation of<br />
H3K27 in cells more strongly<br />
than EZH2 selective inhibitors.<br />
Valemetostat also displayed<br />
antitumour activity in various<br />
haematological malignancies<br />
in preclinical models.<br />
Sakigake Designation<br />
was granted based on the<br />
May 2019 / FUTURE MEDICINE / 33
Olaparib to treat advanced<br />
breast cancer in EU<br />
The European Commission has<br />
approved olaparib (Lynparza) as<br />
monotherapy for the treatment of adult<br />
patients with germline<br />
BRCA1/2-mutations (gBRCAm), and<br />
who have human epidermal growth<br />
factor receptor 2 (HER2)-negative<br />
locally-advanced or metastatic breast<br />
cancer.<br />
Patients receiving the therapy<br />
should have previously been treated<br />
with an anthracycline and a taxane in<br />
the (neo)adjuvant or metastatic setting<br />
unless they were unsuitable for these<br />
treatments. Patients with hormone<br />
receptor (HR)-positive breast cancer<br />
should also have progressed on or<br />
after prior endocrine therapy or be<br />
considered unsuitable for endocrine<br />
therapy.<br />
The approval was based on data<br />
from the randomised, open-label, phase<br />
III OlympiAD trial which tested olaparib<br />
vs. physician’s choice of chemotherapy<br />
(capecitabine, eribulin, or vinorelbine).<br />
In the trial, olaparib provided patients<br />
with a statistically-significant median<br />
progression-free survival improvement<br />
of 2.8 months.<br />
This is the third indication for<br />
olaparib in the EU, said AstraZeneca<br />
and MSD.<br />
preliminary results of an<br />
ongoing phase 1 study<br />
assessing the safety and<br />
efficacy of valemetostat in<br />
patients with non-Hodgkin<br />
lymphomas (NHL) including<br />
PTCLs, which were presented<br />
at the 2017 annual meeting<br />
of the American Society of<br />
Hematology (ASH).<br />
The Sakigake Designation<br />
System promotes R&D in<br />
Japan, driving early practical<br />
application for innovative<br />
pharmaceutical products,<br />
medical devices, and<br />
regenerative medicines.<br />
Dolutegravir<br />
+ lamivudine<br />
combo for HIV1<br />
The US FDA approved<br />
dolutegravir and<br />
lamivudine (Dovato) as a<br />
complete regimen for the<br />
treatment of HIV-1 infection<br />
in adults with no antiretroviral<br />
treatment history.<br />
This is the first FDAapproved<br />
two-drug, fixed-<br />
dose, complete regimen for<br />
HIV-infected adults who<br />
have never received<br />
treatment for HIV, ViiV<br />
Healthcare said.<br />
The efficacy and safety<br />
of Dovato, one tablet taken<br />
daily, were demonstrated in<br />
two identical, randomized,<br />
double-blind, controlled<br />
clinical trials in 1,433 HIVinfected<br />
adults with no prior<br />
antiretroviral treatment<br />
history.<br />
The trials showed that<br />
a drug regimen containing<br />
dolutegravir and lamivudine<br />
had a similar effect of<br />
reducing the amount of<br />
HIV in the blood compared<br />
to another drug regimen,<br />
which included dolutegravir,<br />
emtricitabine, and tenofovir.<br />
Fast track for<br />
oral tebipenem<br />
to treat UTI <br />
The US FDA has granted<br />
Fast Track designation to<br />
SPR994, an oral carbapenem<br />
for complicated UTI and<br />
acute pyelonephritis.<br />
SPR994 is tebipenem<br />
pivoxil hydrobromide —<br />
an oral formulation of<br />
tebipenem, an antibiotic in<br />
the carbapenem class.<br />
The preclinical models of<br />
SPR994 have already shown<br />
its efficacy against gram<br />
negative bacteria including<br />
E. coli, producing extendedspectrum<br />
beta-lactamase<br />
(ESBLs) and ESBLs producing<br />
Klebsiella pneumoniae,<br />
similar to IV ertapenem.<br />
The drug has been<br />
already given Qualified<br />
Infectious Disease Product<br />
(QIDP) designation.<br />
Completion of phase<br />
1, double-blind, placebocontrolled,<br />
ascending dose,<br />
a multi-cohort study has<br />
enabled dose selection for<br />
the company’s upcoming<br />
phase 3 trial, ADAPT-PO.<br />
This will be a randomized,<br />
double-blind, double-dummy,<br />
multicentre, prospective<br />
study designed to assess<br />
the efficacy, safety, and<br />
pharmacokinetics of<br />
tebipenem vs intravenous (IV)<br />
ertapenem in patients with<br />
complicated UTI or AP.<br />
Dacomitinib to<br />
treat patients<br />
with NSCLC<br />
in EU<br />
Pfizer Inc. announced that<br />
the EC has approved<br />
34 / FUTURE MEDICINE / May 2019
under the Priority Review<br />
programme.<br />
Romosozumab<br />
to treat<br />
osteoporosis<br />
Amgen and UCB<br />
announced that the<br />
US FDA has approved<br />
romosozumab-aqqg<br />
(Evenity) for the treatment<br />
of osteoporosis in<br />
postmenopausal women at<br />
high risk for fracture.<br />
Romosozumab-aqqg<br />
has a dual effect that both<br />
increases bone formation and<br />
to a lesser extent reduces<br />
bone resorption. A full course<br />
of the therapy is 12 monthly<br />
doses.<br />
The FDA based its<br />
approval of romosozumabaqqg<br />
on the results of two<br />
phase 3 studies. In the<br />
placebo-controlled FRAME<br />
study, treatment with<br />
dacomitinib (Vizimpro), a<br />
tyrosine kinase inhibitor<br />
(TKI), as monotherapy for<br />
the first-line treatment<br />
of adult patients with<br />
locally advanced or<br />
metastatic non-small cell<br />
lung cancer (NSCLC) with<br />
epidermal growth factor<br />
receptor (EGFR)-activating<br />
mutations.<br />
The EC approval of<br />
dacomitinib was supported<br />
by data from ARCHER 1050,<br />
a randomized, multicentre,<br />
multinational, open-label,<br />
phase 3 study conducted in<br />
patients with unresectable,<br />
metastatic or recurrent<br />
NSCLC, harbouring EGFR<br />
exon 19 deletion or exon 21<br />
L858R substitution<br />
mutations.<br />
Dacomitinib is also<br />
approved in the US for the<br />
first-line treatment of patients<br />
with metastatic NSCLC with<br />
EGFR exon 19 deletion or<br />
exon 21 L858R substitution<br />
mutations as detected<br />
by an FDA-approved test.<br />
Additionally, it is approved<br />
in Japan for EGFR gene<br />
mutation-positive, inoperable<br />
or recurrent NSCLC, and<br />
in Canada for the first-line<br />
treatment of adult patients<br />
with unresectable locally<br />
advanced or metastatic<br />
NSCLC with confirmed<br />
EGFR exon 19 deletion or<br />
exon 21 L858R substitution<br />
mutations. The applications<br />
in the US and Japan were<br />
reviewed and approved<br />
Cladribine as oral treatment for<br />
multiple sclerosis<br />
Cladribine (Mavenclad) has been granted approval<br />
to treat relapsing forms of multiple sclerosis (MS) in<br />
adults by the USFDA.<br />
Cladribine is not recommended for MS patients<br />
with clinically isolated syndrome. Because of its safety<br />
pro<strong>file</strong>, the use of the drug is generally recommended<br />
for patients who have had an inadequate response to, or<br />
are unable to tolerate, an alternate drug indicated for the<br />
treatment of MS.<br />
The efficacy of cladribine was shown in a clinical<br />
trial in 1,326 patients with relapsing forms of MS who<br />
had at least one relapse in the previous 12 months. The<br />
drug significantly decreased the number of relapses<br />
experienced by these patients compared to placebo.<br />
Cladribine also reduced the progression of disability<br />
compared to placebo.<br />
romosozumab-aqqg<br />
resulted in a significant<br />
reduction of new vertebral<br />
fracture at 12 months<br />
compared to placebo.<br />
This significant reduction<br />
in fracture risk persisted<br />
through the second year in<br />
women who received the<br />
drug during the first year and<br />
transitioned to denosumab<br />
compared to those who<br />
transitioned from placebo to<br />
denosumab.<br />
In addition,<br />
romosozumab-aqqg<br />
significantly increased bone<br />
mineral density at the<br />
lumbar spine, total hip and<br />
femoral neck compared<br />
to placebo at 12 months.<br />
Following the transition<br />
from romosozumab-aqqg<br />
to denosumab at month 12,<br />
BMD continued to increase<br />
through month 24.<br />
In the active-controlled<br />
ARCH study, treatment<br />
with romosozumab-aqqg<br />
for 12 months followed by<br />
12 months of alendronate<br />
significantly reduced the<br />
incidence of new vertebral<br />
fracture at 24 months.<br />
May 2019 / FUTURE MEDICINE / 35
straight talk<br />
“WE STILL DON’T HAVE A<br />
CLEAR UNDERSTANDING<br />
OF THE ACTUAL DISEASE<br />
BURDEN IN INDIA”<br />
VIJAY CHANDRU, Co-Founder and<br />
Director, Strand Life Sciences, and INAE<br />
Distinguished Technologist at Indian<br />
Institute of Science, is an academic<br />
turned entrepreneur. He got his PhD<br />
in mathematics of decision sciences at<br />
MIT in 1982. He taught and conducted<br />
research in computational mathematics of<br />
optimization, geometry, logic and biology<br />
at Purdue University (1982-1993) and<br />
the Indian Institute of Science (1992-<br />
2005). The vision that drove him to<br />
entrepreneurship was to create successful,<br />
world-class, technology innovation<br />
companies out of India. In 1998, he joined<br />
hands with kindred spirits to create the<br />
Simputer (a handheld computer). He also<br />
co-founded PicoPeta Simputers, which<br />
commercialized the Simputer to create<br />
the Amida Simputer. Strand Life Sciences<br />
started out as an exciting life science<br />
informatics company based in Bangalore,<br />
India. Strand, which has captured around<br />
30% of the global market for analytics<br />
software in research biology for the<br />
various “omics” - genomics, proteomics,<br />
metabolomics, etc., is a global, personalized<br />
medicine company that uses clinical<br />
genomics for oncology and inherited<br />
disorders. He also helped create non-profit<br />
agencies that work for neglected disease<br />
communities. In an exclusive interview<br />
with Editor CH UNNIKRISHNAN, Chandru,<br />
who is pursuing the vision of affordable<br />
precision medicine, says that India needs<br />
to do a lot more to have clarity on its<br />
actual disease burden, especially rare and<br />
undiagnosed diseases. Edited excerpts:<br />
India is one of the large geographies where the burden<br />
of rare and undiagnosed diseases, mainly linked to genetic<br />
disorders, is high with a prevalence of around 70 million.<br />
But the country still doesn’t have accurate data or a<br />
registry to formulate appropriate policies or a budget to<br />
take care of the patients. Why?<br />
We still don’t have a clear understanding of the actual<br />
disease burden in the country, especially in case of the rare<br />
and undiagnosed diseases linked to genetic causes. I think<br />
the challenge here is that there has been no systematic<br />
surveying. As per information from government agencies,<br />
at least all the diseases that have been given the disability<br />
status will show up in census data by the 2020-21 survey,<br />
which will start giving us some clue. With this, at least major<br />
disabilities like hemophilia, thalassemia, sickle cell anaemia,<br />
muscular dystrophy, multiple sclerosis and perhaps a few<br />
more will be captured and those numbers will be known<br />
with better accuracy. Secondly, there is also a hope that<br />
because there are a lot of patient communities, awareness<br />
activities, camps, etc. happening now, that will help slowly<br />
build the data, as these platforms will be able to provide<br />
useful information and numbers.<br />
ICMR has announced that it will build a registry of<br />
rare diseases in India. We will have to wait and see how<br />
successful that effort will be. Though the apex medical<br />
research body was claimingly successful in building India’s<br />
cancer registry, the numbers still do not seem very<br />
accurate. While the cancer registry shows about 1.3 million<br />
new cases per year, there seems to be something wrong<br />
with this number. For instance, China, which has almost the<br />
same level of disease burden as India, had recently declared<br />
its data of new cancer patients as 4.1 million per year. So,<br />
apparently, the data claimed by the Indian<br />
registry has probably underestimated the prevalence.<br />
ICMR is using some kind of sampling technique and then<br />
extrapolating it. But for some reason, this number seems<br />
conservative, I feel.<br />
On rare diseases, it’s true that some pharma companies<br />
36 / FUTURE MEDICINE / May 2019
Vijay Chandru<br />
PHOTO: UMESH GOSWAMI<br />
have somewhat better-estimated data since<br />
they have information that is collected<br />
from doctors or directly from the market.<br />
But when you ask these companies<br />
about lysosomal storage disorders, they<br />
estimate it as a few hundreds. Similarly,<br />
many haematologists estimate thalassemia<br />
cases at 1 to 1.5 lakh patients. So, where is<br />
this number of 70 million patients of rare<br />
diseases that are projected at every forum<br />
coming from, I often wonder. China has<br />
published that their population with rare<br />
diseases as 20 million. I agree that India<br />
has more consanguineous marriage within<br />
the same community, among other factors,<br />
that cause genetic disorders. But still, such<br />
a huge difference! So, we don’t know what<br />
the numbers are. But I feel there is plenty<br />
Where is this<br />
number of<br />
70 million<br />
patients of rare<br />
diseases that<br />
are projected<br />
at every forum<br />
coming from, I<br />
often wonder.<br />
of work to be done to have a clarity on the<br />
actual disease burden. These numbers are<br />
important to formulate our priorities,<br />
where funds should be given and what<br />
sort of facilities have to be created, among<br />
others.<br />
There are government bodies like IGIB<br />
and ICMR, private companies and charity<br />
organisations working to address patient<br />
needs. At what stage are these works at<br />
present, any idea?<br />
As far as rare diseases are concerned,<br />
some areas have seen better progress.<br />
For example, patient tracking and<br />
diagnosis in haematological disorders are<br />
comparatively better organised and there<br />
is a lot of work currently going on. While<br />
May 2019 / FUTURE MEDICINE / 37
that the allocation of the budget is not possible unless the<br />
government knows the burden.<br />
But I think it is just a matter of time; It will happen and<br />
the good thing is that the country is looking at healthcare<br />
much more seriously now and it is also an era when medical<br />
records are largely being digitized. Even for rare diseases,<br />
the country is looking much more carefully at the data and<br />
digital records from various sources.<br />
What about the therapy side?<br />
The therapy side is always a challenge. There are many<br />
therapies that are used for the maintenance of patients.<br />
Those therapies, I think, patient communities and hospitals<br />
are tracking and providing. But even then, we don’t have<br />
a comprehensive list of medicines. Rather, none has put<br />
together a good collection of information about medicines<br />
that the patients would need for rare diseases. By this,<br />
what I am referring to is the kind of a comprehensive<br />
list that India prepared on essential medicines, that the<br />
country’s physicians would require along with all other<br />
related information about availability, pricing of generics and<br />
patented products and so on, when the country was about<br />
to sign the Dunkel Draft. Such a list needs to be prepared<br />
for rare diseases as well, detailing the use, cost, availability<br />
and accessibility. Such an effort will help in ensuring the<br />
availability and accessibility of treatment for Indian patients<br />
in case of rare diseases. Otherwise, a large section of the<br />
population will still be left untreated as most of the targeted<br />
treatments currently available in the market is beyond the<br />
reach of the mass.<br />
activities for muscular disorders is slowly<br />
picking up, other segments like primary<br />
immuno-deficiency and others are still<br />
poorly covered and there is very little<br />
help for patients at present. Overall, I think<br />
we have a fundamental issue of really<br />
starting to think about evidence-based<br />
planning for the care of rare diseases,<br />
moving away from the eminence-based<br />
planning.<br />
I remember, the health Secretary of<br />
Karnataka once asked us (the industry),<br />
while the state was proposing a draft on its<br />
health policy, if we can provide data about<br />
the actual burden and he made it a point<br />
The genetic study of the Indian population, which is<br />
essential to address rare diseases, is still minuscule. What<br />
needs to be done to expand it?<br />
There are some broad-based genetic studies that are<br />
being planned as part of projects like Genome India and so<br />
on. But there are also more focused cohort studies such as<br />
the neurological disorder study that is done by NIMHANS<br />
etc., going on in parallel. So, it has slowly started to build up,<br />
but the volume of work is yet to pick up. However, the good<br />
thing is that the country has the capacity now.<br />
Large sequencers have been set up at least in five to six<br />
locations in the country. So, the ability to run the sequences<br />
to do the bioinformatics has been built and we are in a<br />
position to do it. But if you ask me if we have done it, I would<br />
say it is not very visible. Though a few companies, including<br />
Strand Life Sciences and MedGenome, have probably<br />
generated a fair amount of data, I don’t think academic<br />
groups have done as much. However, since capacities have<br />
been built, they seem to be getting adequately funded<br />
shortly. Going forward, we will see better, and more data<br />
being generated as things are moving in the right direction.<br />
Rare diseases are definitely on the agenda and it is time to<br />
think positive.<br />
38 / FUTURE MEDICINE / May 2019
N.Z. POLO ASSN.<br />
SINCE 1936<br />
MEMO PAD<br />
Gift Voucher<br />
To ....................................................<br />
From ....................................................<br />
Code<br />
Date: ..................................<br />
Validity - 6 months<br />
www.ebuydeals.co.in<br />
Terms & Condition<br />
1) To redeem your gift voucher, simply enter the code at the time of checkout.<br />
2) Courier Charges as per mention in website. i.e: www.ebuydeals.co.in<br />
3) Vouchers without holograms will not be accepted.<br />
4) Scratched, Mutilated or defaced voucher will not be accepted.<br />
5) Please check the goods against delivery.<br />
6) Max 30 GV’s can be redeemed on single invoice.<br />
7) Any dispute arising is subject to Mumbai Jurisdiction.<br />
8) Not applicable on Red Hot Sale/End of Season Sale.<br />
9) These gift vouchers will not be applicable on Bank offers.<br />
10) If purchase value exceed the value of the voucher then differential amount should be paid by Card or COD.<br />
11) This voucher is redeemable only by the bearer. No replacement/compensation is permissible for lost vouchers.<br />
12) Ebuy Deals reserves the right to alter the terms & conditions of this Gift Voucher.<br />
13) Once the voucher is used, Ebuy Deals will not entertain any request for cancellation.<br />
14) Kindly check the product when it is delivered because we are not responsible after delivery.<br />
15) These Vouchers will not be valid on other vouchers OR Classic Category product being sold on the website.<br />
16) You will not get any refund OR Balance carried forward OR cash back for Excess value of Voucher amount used.
case reports<br />
ROBOTIC BYPASS<br />
A unique case where the least invasive option of minimally invasive<br />
cardiac surgery is employed<br />
Mrs Malliga, a 63-year old lady from Ambattur in<br />
Chennai, had been suffering from chest pain for the<br />
past six months. As her condition worsened and<br />
the chest pains increased, she was brought to<br />
Apollo Hospital Chennai, where she consulted with Dr.<br />
Mohammed M Yusuf, a cardiothoracic surgeon specializing<br />
in Minimally Invasive Cardiac Surgery and Dr Damodharan,<br />
Senior Consultant Cardiologist. Mrs Malliga complained of<br />
chest pain on minimal exertion. Her case history<br />
showed that she was diabetic and had slightly higher<br />
cholesterol levels than normal, two high-risk factors for<br />
heart disease.<br />
An angiography revealed blocks in three major arteries<br />
that supply blood to the heart. Typically in such cases, an<br />
open-heart bypass surgery or stents would be indicated.<br />
Keeping the history of diabetes and cholesterol in mind, an<br />
open-heart bypass surgery was deemed the best treatment<br />
option. This procedure involves using a healthy blood vessel<br />
from another part of the body to allow blood to bypass the<br />
block in the vessel. For blocks in the left anterior descending<br />
artery, a healthy artery from the chest wall itself is preferred.<br />
However, for other less important arteries, surgeons <strong>may</strong> use<br />
veins from the leg as well.<br />
“A bypass surgery is more effective for blocks in the<br />
major arteries, compared with stents. The majority of stents<br />
are beneficial for up to 5-10 years, after which blocks <strong>may</strong><br />
reappear”, says Dr Yusuf. While the treatment outcome is<br />
excellent and mortality rates are extremely low in bypass<br />
surgeries, the procedure involves making a 6-8 inch long<br />
incision in the chest and cutting through the breastbone to<br />
access the heart, resulting in a considerably long recovery<br />
period that can last up to 4 months.<br />
Recent advances in open-heart surgery techniques<br />
include minimally invasive approaches that require smaller<br />
incisions and offer faster recovery periods. Instead of the<br />
large 6-8 inch incision for the conventional bypass surgery, a<br />
smaller cut in the chest is sufficient for a minimally invasive<br />
procedure. This reduces morbidity and brings the recovery<br />
time to 2 weeks. Dr Yusuf and his medical team have been<br />
performing minimally invasive bypass surgeries for over a<br />
year with excellent results.<br />
More recently, robotic assistance to perform the<br />
procedure has gained interest. Dr Yusuf and his team opted<br />
to conduct this minimally invasive operation using robotic<br />
assistance rather than the conventional<br />
approach. Two of the three major blood<br />
vessels of the patient were severely blocked<br />
and required bypass surgery. The blocks<br />
in other vessels could be treated with<br />
angiography stent insertion. Dr Yusuf’s<br />
team, therefore, decided on a 2-step<br />
approach. Under the guidance of Dr Frank<br />
Vanpraet, Director of Robotic and Minimally<br />
Invasive Cardiac Surgery, OLV Hospital, Aalst,<br />
Belgium, Dr Yusuf performed the minimally<br />
invasive coronary artery bypass grafting<br />
surgery using robotic assistance. In the<br />
ROBOT-ASSISTED MINIMALLY<br />
INVASIVE CORONARY ARTERY<br />
BYPASS TECHNIQUE LOWERS<br />
THE RISK OF COMPLICATIONS<br />
SUCH AS STROKE, EXCESSIVE<br />
BLOOD LOSS, AND INFECTION<br />
second step, two days after the first<br />
surgery, Dr Damodharan performed<br />
angiography and drug-eluting stent insertion<br />
for the remaining blocks in Mrs Malliga’s<br />
coronary arteries. This was done with the<br />
assistance of a specialized intravascular<br />
ultrasound.<br />
Robot-assisted minimally invasive<br />
coronary artery bypass grafting has several<br />
advantages, as it is the least invasive<br />
option among minimally invasive cardiac<br />
surgeries. This technique lowers the risk<br />
of complications such as stroke, excessive<br />
blood loss, and infection. Patients also<br />
have reduced pain, smaller scars and<br />
faster recovery periods and it is ideal<br />
for multivessel blocks. “Even though it is<br />
more expensive than conventional bypass<br />
40 / FUTURE MEDICINE / May 2019
surgeries, it should be recommended for young patients<br />
who would like to get back to work immediately and in<br />
high-risk patients with other comorbidities”, says Dr Yusuf.<br />
With our lifestyle changes, more and more young people<br />
are suffering from heart conditions and such quick recovery<br />
treatment approaches are likely to be more economical in<br />
the long run.<br />
Robotic Minimally Invasive Hybrid Revascularization is<br />
already being performed in countries such as Japan, United<br />
Kingdom and the United States. However, this was the first<br />
instance of the minimally invasive approach with robotic<br />
assistance in India.<br />
The multiple step approach used for<br />
Mrs Malliga showed excellent results as<br />
she was discharged in 48 hours after the<br />
procedure and has since returned to full<br />
normal activity. She will, of course, be<br />
regularly followed up to ensure that no new<br />
complications arise.<br />
DR SHIVANEE SHAH<br />
May 2019 / FUTURE MEDICINE / 41
case reports<br />
ECMO RESCUE<br />
Extracorporeal membrane oxygenation must be initiated for ARDS<br />
as soon as standard therapy starts failing<br />
Nagesh, a middle-class executive<br />
at a local shopping mall in Bengaluru, was<br />
34-year-old<br />
suffering from fever and severe shortness of<br />
breath and was admitted to St. John’s Hospital,<br />
Bengaluru. His clinical history indicated no comorbidities or<br />
other illnesses; however, he had recently travelled to<br />
another city.<br />
As his condition was worsening, he was diagnosed with<br />
severe community-acquired pneumonia and admitted to<br />
the intensive care unit where he was started on anti-flu<br />
medicines and placed on a mechanical<br />
ventilator to help with breathing. However,<br />
his oxygen parameters did not improve<br />
even after artificial ventilation in the prone<br />
position after sixteen hours. Doctors at<br />
St. John’s recommended extracorporeal<br />
membrane oxygenation (ECMO) support as<br />
a last, life-saving resort. ECMO is a medical<br />
device used to supply oxygen to circulating<br />
blood through an oxygenator in an effort<br />
42 / FUTURE MEDICINE / May 2019
to bypass the requirement of an injured or non-functional<br />
lung.<br />
Unfortunately, most hospitals do not have ECMO facilities<br />
in India and St John’s hospital is no exception. The medical<br />
team at St. John’s therefore reached out to Narayana Health<br />
City, Bengaluru, for assistance. Ideally, Nagesh would be<br />
transferred to Narayana Health City before ECMO treatment.<br />
However, his condition was critical, and he was not able to<br />
be transported. In a life-saving move, the 12-member ECMO<br />
team led by Dr Harish M M, consultant and in-charge of the<br />
multi-disciplinary intensive care unit, Narayana Health City,<br />
came to St John’s with a portable ECMO unit, initiated venovenous<br />
ECMO at St John’s, and then transferred Nagesh to<br />
Narayana Health City while on ECMO support.<br />
At Narayana Health City, Nagesh underwent a repeat<br />
X-ray, while his previous treatment regime of Tamiflu and<br />
antibiotics was continued along with the ECMO. Because of<br />
his deteriorating condition in terms of clinical presentation<br />
with bilateral infiltration and a low response to ventilation<br />
support, he was considered to be a case of severe acute<br />
respiratory distress syndrome (ARDS). A tracheal aspirate<br />
was used for molecular testing using polymerase chain<br />
reaction to identify the causative agent for the respiratory<br />
illness. Molecular testing has the benefit of being rapid as<br />
well as having high sensitivity, and within 24 hours, he was<br />
confirmed with H1N1 infection.<br />
ARDS is a severe form of lung injury and is defined based<br />
on the development of acute dyspnea and hypoxemia within<br />
7 days after the insult, along with radiographic changes<br />
showing bilateral infiltrates. ARDS can be classified as mild<br />
to severe, depending on the ratio of the partial pressure of<br />
oxygen in the patient’s arterial blood (PaO2) to the fraction<br />
of oxygen in the inspired air (FiO2). If this is ratio is less than<br />
100, the patient is classified as suffering from severe ARDS.<br />
Nagesh’s PaO2/FiO2 ratio was less than 100, classifying him<br />
as having severe ARDS.<br />
While there is no specific therapy for ARDS, treatment<br />
should depend on the underlying condition, along with<br />
appropriate supportive care and artificial ventilation. Nagesh<br />
was continued on Tamiflu and ECMO and a broad-spectrum<br />
antibiotic to prevent bacterial infections during ventilation<br />
support.<br />
The decision to remove ECMO support is dependent on<br />
the oxygen levels as well as the general condition of the<br />
patient. Absence of high-grade fever and infections, normal<br />
electrolytes, a normal heart rate and brain function are<br />
all factors indicating whether the patient can be weaned<br />
of the ECMO support. Though blood culture is a definitive<br />
indicator of infection, its yield is very poor especially when<br />
patients are already on antibiotics. Other indirect indicators<br />
of infection, including WBC count, body temperature,<br />
and infiltration in the lungs are monitored daily, whereas<br />
biomarkers like procalcitonin, C reactive protein etc.<br />
are monitored every 2-4 days. Once these biochemical<br />
parameters return to normal, the patient<br />
can be weaned off ECMO. Even after<br />
discharge, sequelae of ARDS, such as<br />
weight loss, fatigue, functional compromised<br />
life, myopathy etc. can often linger for<br />
months.<br />
Nagesh had to be supported on ECMO<br />
for 12 days before his PaO2/FiO2 ratio<br />
started to stabilise and his lung function<br />
returned to normal. After discharge, he was<br />
continued on multivitamins, and followed<br />
up after 1 week. Luckily, he did not have to<br />
witness prolonged effects of ARDS and he<br />
returned to normalcy at the 1-week follow<br />
up.<br />
According to Dr Harish, ECMO comes<br />
with a high cost, running into a few lakhs.<br />
However, the most important criteria for<br />
THE MOST IMPORTANT<br />
CRITERIA FOR STARTING<br />
ECMO THERAPY IS THAT THE<br />
PATHOLOGY SHOULD BE<br />
REVERSIBLE<br />
starting ECMO therapy is that the pathology<br />
should be reversible. It is not recommended<br />
for severe stroke or advanced cancer<br />
patients with ARDS, who <strong>may</strong> already be<br />
bedridden. Side effects of ECMO include<br />
bleeding, air embolism, low platelet counts<br />
(platelets <strong>may</strong> be destroyed in the external<br />
tubes), and hemolysis. Platelet or blood<br />
transfusions <strong>may</strong> often be required to<br />
address platelet/blood loss.<br />
Despite the costs and side effects,<br />
ECMO must be initiated early once standard<br />
therapy (including prone ventilation) for<br />
ARDS fails, as ARDS will not respond to<br />
ECMO once the lung moves to a fibrotic<br />
phase. While few hospitals have ECMO<br />
facilities, Dr Harish advocates that patients<br />
should be referred to a suitable health care<br />
centre as early as possible and definitely<br />
no later than 2 sessions of ineffective<br />
ventilation in prone position.<br />
DR SHIVANEE SHAH<br />
May 2019 / FUTURE MEDICINE / 43
case reports<br />
A<br />
AVOIDING<br />
AN AORTIC<br />
CATASTROPHE<br />
Should an ECG rule out a heart attack, consider performing an echo<br />
to check for aortic dissection<br />
Satish (name changed) suddenly<br />
started having chest pains and was rushed to a<br />
55-year-old<br />
local hospital in Pallakad, Kerala. As an ex-army<br />
man, he had never experienced such pain and his family<br />
assumed he was having a heart attack. However, all initial<br />
tests that were run were negative and an echo cardiography<br />
was performed. The echo showed an aortic dissection and<br />
Satish was transferred to Amrita Hospital, Kochi, which has a<br />
dedicated Centre for Aortic Diseases and Marfan Syndrome.<br />
Here he was immediately rushed to the operation theatre<br />
without further ado by the medical team led by Dr Praveen<br />
Varma, Head of Cardiovascular and Thoracic Surgery.<br />
An aortic dissection is a tear in the inner lining of<br />
the aorta, just above the aortic valve, and results due to<br />
increased stress within the walls of the aorta. Type A aortic<br />
dissection such as this one is a life-threatening medical<br />
emergency requiring immediate surgery, without which<br />
mortality can be almost 100%. Once the aorta dilates from<br />
the normal range of 2.5-3 cm to 4.5-5 cm, it is likely to<br />
dissect. Right before the team started operating on Satish,<br />
his aortic wall ruptured outside However, the procedure was<br />
already underway, and the medical team remained unfazed<br />
as they proceeded with the aortic root<br />
replacement surgery, which is a complex<br />
surgery often taking 8-10 hours.<br />
This procedure involves multiple steps.<br />
The first step involves a surgical technique<br />
called total circulatory arrest in which the<br />
body is cooled to less than 18°C before<br />
cutting off blood circulation to the whole<br />
body. The cool temperatures allow the brain<br />
to survive the circulatory arrest, however,<br />
brain activity must be closely monitored<br />
to ensure that the cool temperatures keep<br />
the brain quiescent throughout the surgery.<br />
This is critical while performing delicate<br />
procedures on large blood vessels such as in<br />
this case. The patient is typically connected<br />
to a cardiopulmonary bypass, an external<br />
heart-lung machine, which drains the blood<br />
out of the body and cools it to less than 18<br />
°C. Brain activity is monitored by monitoring<br />
blood flow to the brain by Near Infrared<br />
Spectroscopy. Once the brain function has<br />
44 / FUTURE MEDICINE / May 2019
stopped, the actual aortic root replacement surgery can<br />
commence. This was done following the Bentall procedure,<br />
in which the aortic valve, the aortic root and the ascending<br />
aorta are replaced with a composite graft, and the coronary<br />
arteries are then re-implanted into the new graft. Once the<br />
replacement surgery is completed, the blood is warmed<br />
again, and the patient is then returned to normal blood flow<br />
and brain activity.<br />
Satish’s surgery went well, and he was kept in the ICU<br />
for 2 days and discharged 8 days post-surgery. Now he is<br />
on strong anti-hypertensive drugs, which will be his lifelong<br />
companions. He is also given a blood thinner to ensure the<br />
proper functioning of the new artificial mechanical valve.<br />
While aortic dissection and rupture are relatively<br />
uncommon, major risk factors are uncontrolled high blood<br />
pressure, atherosclerosis, cocaine abuse, bicuspid aortic<br />
valve, or genetic diseases such as Turner’s syndrome or<br />
Marfan syndrome. Patients with Turner’s syndrome have<br />
a missing or structurally altered X-chromosome resulting<br />
in many abnormalities including aortic valve defects.<br />
Marfan syndrome is caused due to an autosomal dominant<br />
mutation in the FBN1 gene which encodes a protein called<br />
fibrillin 1. This protein is an extracellular matrix glycoprotein<br />
that provides structural support to connective tissue<br />
throughout the body. Patients with Marfan syndrome have<br />
typical visual clinical features of elongated limbs, fingers<br />
and toes, and scoliosis. Marfan syndrome <strong>may</strong> also affect<br />
other organs including eyes, bones, lungs, and heart. Marfan<br />
syndrome patients are at a high risk of aortic dilation, aortic<br />
aneurysm and mitral valve prolapse, and aortic dissection<br />
which <strong>may</strong> well be the most serious of all<br />
the complications associated with Marfan<br />
syndrome.<br />
In 2016, a special Centre for Aortic<br />
Diseases and Marfan Syndrome was<br />
started at Amrita Hospital, Kochi. This<br />
is the only such center in India that is<br />
dedicated to patients with Marfan syndrome<br />
and other aortic diseases. It comprises<br />
of a multidisciplinary team including<br />
cardiologists, surgeons, ophthalmologists,<br />
geneticists, obstetricians as well as<br />
radiologists. These doctors together offer<br />
comprehensive care including genetic<br />
counselling and associated medical support<br />
as required. The cardiovascular surgeons<br />
are skilled to deal with the most serious<br />
complications like aortic ruptures. Today<br />
WHILE AORTIC DISSECTION<br />
AND RUPTURE ARE RELATIVELY<br />
UNCOMMON, MAJOR RISK<br />
FACTORS ARE UNCONTROLLED<br />
HIGH BP, ATHEROSCLEROSIS,<br />
COCAINE ABUSE, BICUSPID<br />
AORTIC VALVE, OR GENETIC<br />
DISEASES SUCH AS TURNER’S<br />
SYNDROME<br />
over 50 patients are registered with this<br />
clinic and undergo regular screenings every<br />
6 months. In case clinical observations<br />
indicate that the aorta is dilating, elective<br />
aortic root replacement <strong>may</strong> also be offered.<br />
According to Dr Varma, even though<br />
aortic diseases and acute aortic syndromes<br />
are more catastrophic and complicated,<br />
they are not given much importance in India<br />
and are often overlooked and misdiagnosed<br />
as heart attacks. Should an ECG rule out a<br />
heart attack, consider performing an echo<br />
to check for aortic dissection. Patients with<br />
aortic dissection should immediately be<br />
transferred to the nearest hospital with<br />
surgical expertise.<br />
DR SHIVANEE SHAH<br />
May 2019 / FUTURE MEDICINE / 45
case reports<br />
SILENT THALASSEMIA<br />
Alpha thalassemia is under-reported in India because of the need for genetic<br />
testing to confirm the blood disorder<br />
Two-and-a-half-year-old Pratik (name changed), a<br />
normal, seemingly healthy child, was visiting his<br />
paediatrician for a regular check-up. The paediatrician<br />
thought that Pratik was pale looking and requested a<br />
complete blood count investigation to be done. The results<br />
indicated low haemoglobin levels, high RBC counts, and<br />
high red cell distribution width. In addition, cells were small,<br />
indicative of microcytic hypochromic anaemia. Pratik was<br />
therefore suspected to have iron deficiency anaemia and<br />
serum iron studies were done. These also showed evidence of<br />
iron deficiency and Pratik was started on iron supplements. At<br />
the one month follow up, his repeat complete blood counts<br />
showed improved haemoglobin levels. However, the RBC<br />
count was still high and the microcytosis was severe. He was<br />
referred to Dr Swati Kanakia, Paediatric Hemato-Oncologist,<br />
Lilavati Hospital and Research Center, Mumbai. Based on<br />
complete blood count results, Dr Kanakia suspected Pratik<br />
<strong>may</strong> have thalassemia minor.<br />
Thalassemia is a genetic disorder in which<br />
the body produces low levels of functional<br />
haemoglobin. Haemoglobin is made up of<br />
alpha and beta chains. Depending on which<br />
protein levels are affected, a patient can have<br />
either alpha thalassemia or beta thalassemia.<br />
BASED ON THE SEVERITY<br />
OF CLINICAL FEATURES,<br />
ALPHA THALASSEMIA<br />
CAN BE CLASSIFIED AS A<br />
SILENT CARRIER<br />
Based on the severity of clinical features,<br />
alpha thalassemia can be classified as a silent<br />
carrier, thalassemia trait, haemoglobin H<br />
disease, or hydrops fetalis. Beta thalassemia<br />
can be classified as thalassemia minor,<br />
thalassemia intermediate or thalassemia<br />
major. Alpha thalassemia is caused due to<br />
mutations in the HBA1 or HBA2 gene on<br />
chromosome<br />
16, while beta thalassemia is caused due<br />
to mutations in the HBB gene on<br />
chromosome 11.<br />
46 / FUTURE MEDICINE / May 2019
Haemoglobin electrophoresis is a low cost, easily available<br />
test which takes only 24 hours and can rapidly confirm the<br />
diagnosis of beta thalassemia. It is therefore typically done<br />
first. However, alpha thalassemia cannot be detected by this<br />
method and requires genetic testing. Results on Pratik’s blood<br />
sample ruled out beta thalassemia. However, because of the<br />
persistent high RBC count and severe microcytosis, Dr Kanakia<br />
suggested genetic testing to look for mutations in the alpha<br />
haemoglobin chain. Genetic test results revealed a mutation<br />
in the HBA1 gene, confirming that Pratik was a silent carrier<br />
for alpha thalassemia. Similar genetic tests done on Pratik’s<br />
mother showed that she was also a silent carrier. However, her<br />
complete blood count results were absolutely normal, and she<br />
showed no signs of haemoglobin deficiency<br />
or microcytic anaemia. The reason for Pratik’s<br />
iron deficiency turned out to be because of<br />
his diet as he was predominantly on milk. Had<br />
the doctors not pursued the reason for his<br />
microcytic anaemia, Pratik and his mother’s<br />
alpha thalassemia silent carrier status might<br />
have gone undetected.<br />
With genetic testing conveniently<br />
available, screening for thalassemia should be<br />
considered in patients who seem to have iron<br />
deficiency anaemia with microcytic anaemia<br />
and high RBC counts. Correct diagnosis can<br />
prevent incorrect supplementation with<br />
iron and avoid excessive investigation in an<br />
attempt to treat ‘suspected’ refractory iron<br />
deficiency. Further, patients like Pratik, who<br />
are silent carriers, can be counselled so as to<br />
prevent thalassemia cases later.<br />
In India, beta thalassemia is much more<br />
prevalent than alpha thalassemia and is a<br />
major public health concern. Because of the<br />
need for genetic testing to confirm alpha<br />
thalassemia, it is likely that alpha thalassemia<br />
is under-reported as well. “The main goal is to<br />
prevent the birth of children with thalassemia<br />
major”, says Dr Kanakia. If Pratik’s father was<br />
also a carrier for alpha thalassemia, Pratik’s<br />
siblings could have a much more severe<br />
version of thalassemia that <strong>may</strong> require<br />
regular blood transfusions and even bone<br />
marrow transplantation. Treatment can be<br />
very expensive and heavily dependent on<br />
the availability of suitable donors for bone<br />
marrow transplantation. It is therefore<br />
important to screen for carriers of alpha and<br />
beta thalassemia. This will help in identifying<br />
future pregnancies that need to undergo<br />
prenatal testing to rule out severe forms of<br />
thalassemia.<br />
In an effort to aid patient education and<br />
prevent thalassemia major cases, Dr Kanakia<br />
and Kanakia Health Care have created a<br />
programme called Stop Thal, Screening for<br />
Thalassemia and Opting for Prevention. The<br />
programme helps one estimate the chances<br />
of having a completely normal, thalassemia<br />
minor, or thalassemia major child and would<br />
increase awareness amongst thalassemia<br />
patients and prove to be useful in reducing<br />
thalassemia major incidents.<br />
DR SHIVANEE SHAH<br />
May 2019 / FUTURE MEDICINE / 47
column<br />
the cellview<br />
Future of thalassemia care:<br />
An Indian perspective<br />
Screening, along with proper genetic counseling, can help<br />
eradicate the disease<br />
DR RAJANI KANTH<br />
VANGALA<br />
The author is medical<br />
scientist and former<br />
director of SGRF,<br />
Bangalore<br />
In the past few decades, there have<br />
been profound developments in the<br />
management of thalassemia around<br />
the world, as well as in India. Regular<br />
transfusion and iron chelation have<br />
drastically improved the quality of life for<br />
patients and transformed thalassemia from<br />
a fatal disease to a clinically manageable<br />
one. Regular transfusions to maintain pretransfusion<br />
haemoglobin levels to around<br />
9-10g/dl has reduced complications such<br />
as anaemia and compensatory bone<br />
marrow expansion. Repetitive transfusions<br />
lead to iron overload as each transfusion<br />
has 200mg of iron, leading to significant<br />
morbidity and mortality due to damage to<br />
the heart, liver and other organs. Patients in<br />
India develop iron overload complications<br />
like hypogonadism, hypothyroidism,<br />
hypoparathyroidism, diabetes mellitus and<br />
cardiac disorders in the second decade of<br />
life. Iron overload related hepatic diseases<br />
like cirrhosis and fibrosis are very poorly<br />
addressed in India. Some of the major<br />
obstacles leading to high mortality are poor<br />
availability of medical care, lack of safe<br />
and adequate red blood cell transfusions<br />
with high cost, and poor compliance with<br />
chelation therapy. Splenectomy, which is<br />
rare in western countries, is needed for<br />
many patients in India due to insufficient<br />
transfusions. Understanding these<br />
requirements, the major diagnostic and<br />
therapeutic approaches have changed.<br />
Use of MRI scanning to assess iron<br />
overload helps in understanding the<br />
patient condition better to tailor treatments<br />
with reduced co-morbidities. For cardiac<br />
iron overload, non-invasive MRO based<br />
relaxation parameters T2 and T2* are<br />
used frequently. Low T2* indicates high<br />
myocardial iron and is often associated<br />
with poor ventricular function, arrhythmias<br />
and a need for clinical intervention. With<br />
respect to therapies, there are increased<br />
instances of haematopoietic stem cell<br />
transplantation (HSCT) since 1982, as this<br />
gives a better chance of a cure. Even though<br />
the majority of HSCT have been done from<br />
HLA identical related donor, an unrelated<br />
but properly selected match can give good<br />
results too. Recently, more data is being<br />
generated about using cord-blood-based<br />
transplantation. In India, HSCT was not<br />
considered as a life-saving procedure but an<br />
elective option. However, with better quality<br />
of molecular tissue tying, conditioning<br />
regimens and support therapies, it is now<br />
accepted as the better option. As patients<br />
do not need life-long transfusions, it<br />
becomes cost-effective and sustainable. It<br />
is estimated that almost 12,000 children<br />
are born with thalassemia to parents who<br />
are asymptomatic carriers. The carrier<br />
population in India, at about 3.3%, is<br />
significant enough to give importance to<br />
various aspects like prevention too. There<br />
is a need to translate clinical and scientific<br />
data to the population at large to bring<br />
awareness, which in turn can enable<br />
preventive screening. Screening results,<br />
along with proper genetic counseling,<br />
can help eradicate the disease. In small<br />
countries like Cyprus, a near complete<br />
elimination was possible due to mandatory<br />
antenatal testing. We, in our country, have<br />
to evolve with possible mechanisms to<br />
enable people to overcome this disease.<br />
48 / FUTURE MEDICINE / May 2019
Medical<br />
Medical Genetics<br />
Genetics<br />
India’s Most Comprehensive Clinical Exome Test Test<br />
Leveraged by by 550+ Hospitals and and 5500+ 5500+ Clinicians.<br />
MedGenome Clinical Exome Test Test screens<br />
40% more clinically relevant genes<br />
MEDGENOME Competitor Competitor E E Competitor Competitor S<br />
Competitor S Competitor P P<br />
Number of of Genes<br />
Covered<br />
8342 8342<br />
Approx. Approx.<br />
5200 5200<br />
Approx. Approx.<br />
4800 4800<br />
Approx. Approx.<br />
5300 5300<br />
CNV Analysis<br />
Results Validated by by<br />
Sanger Sequencing<br />
CAP CAP Accredited Accredited<br />
(Proficiency<br />
(Proficiency testing)<br />
testing)<br />
Access Access to to FREE FREE Genetic Genetic<br />
Counselling<br />
Counselling<br />
Sign<br />
Sign off<br />
off by<br />
by a<br />
a Clinical<br />
Clinical<br />
Geneticist<br />
Geneticist<br />
Clinically<br />
Clinically relevant<br />
relevant for<br />
for a range<br />
a range of disorders<br />
of disorders across:<br />
across:<br />
Free pre and post test genetic counselling to all patients<br />
Free pre and post test genetic counselling to all patients<br />
Get in touch<br />
Get in touch<br />
1800 1033691<br />
1800 1033691<br />
diagnostics@medgenome.com<br />
diagnostics@medgenome.com<br />
medgenome.com<br />
medgenome.com
medical practice<br />
VIOLENCE GOES<br />
VIRAL<br />
Doctors are the most vulnerable<br />
when it comes to workplace violence<br />
A<br />
research article<br />
published in the<br />
Indian Journal of<br />
Psychiatry claims that<br />
while violence against<br />
doctors is prevalent world<br />
over, Indian doctors face<br />
more violence as compared to those<br />
in western countries. Almost every day,<br />
we hear of patients getting violent<br />
against doctors for one reason or the<br />
other.<br />
This has been attributed to various<br />
factors like poor healthcare budget<br />
allocation by the government, scarcity<br />
of healthcare professionals and<br />
even a lack of training on empathy<br />
and communication in the medical<br />
curriculum.<br />
“It is the junior doctors that bear<br />
the brunt of physical violence by<br />
patients as per research,”<br />
says Dr Jateen Ukrani,<br />
consultant psychiatrist at PsyCare<br />
Neuropsychiatry Centre, Delhi and<br />
one of the authors of the research<br />
paper: Violence against doctors: A viral<br />
epidemic.<br />
He adds that “in emergency rooms,<br />
100% of doctors face some kind of<br />
verbal violence”.<br />
Often, government hospitals are<br />
poorly equipped to deal with the<br />
amount of patient load than what<br />
they actually receive, which makes the<br />
overall experience for the patient and<br />
50 / FUTURE MEDICINE / May 2019
the doctors stressful. This becomes a<br />
fertile soil for breeding violence.<br />
Another factor that contributes<br />
to this growing violence is the lack of<br />
training on effective communication in<br />
the medical curriculum. According to<br />
Dr Indla Ramasubba Reddy, a senior<br />
psychiatrist from VIMHANS Vijayawada,<br />
“Communication and empathy are<br />
not part of the medical curriculum.<br />
These are important for being a good<br />
medical professional. Psychiatrists can<br />
help improve communication skills for<br />
doctors and also help in identifying<br />
early indicators of violence”.<br />
Communication crucial<br />
Experts also point out that with the<br />
advancement of medical science, it has<br />
become more investigation-oriented<br />
which means there is less opportunity<br />
to talk and interact with the patient.<br />
Thus communication suffers, and<br />
patients often don’t understand the<br />
risks. This, combined with all other<br />
factors, has led to a higher rate of<br />
violence against doctors in India. This<br />
leads to many doctors becoming<br />
depressed or quitting the profession<br />
prematurely or not taking risky cases.<br />
“Women doctors are also not<br />
spared from violence and are often<br />
soft targets”, says Dr Varsha Ukrani,<br />
senior psychiatrist from Pandit Madan<br />
Mohan Malviya Hospital, Delhi. It is<br />
pointed out that those working in Obs<br />
& Gyn department are more prone to<br />
violence than any other department.<br />
Dr Vishal Indla, chief psychiatrist<br />
at VIMHANS, Vijayawada says doctors<br />
are the most vulnerable among<br />
all professionals when it comes to<br />
workplace violence due to various<br />
factors ranging from poor budget<br />
allocation for health and weak laws.<br />
The study suggests that though<br />
WOMEN DOCTORS ARE<br />
ALSO NOT SPARED FROM<br />
VIOLENCE AND ARE OFTEN<br />
SOFT TARGETS<br />
acts are in place in 19 states for<br />
protecting doctors against violence,<br />
these are not applied strictly,<br />
and till date, no convictions have been<br />
made. The government should work<br />
on improving the infrastructure and<br />
also on the proper implementation<br />
of these acts, which will reduce the<br />
burden of violence against doctors to<br />
a great extent. Further, doctors are<br />
advised to undergo workshops on the<br />
enhancement of communication<br />
skills and stress management to<br />
deal with such situations. It is also<br />
advised that institutions have a zero<br />
tolerance policy towards violence<br />
against their doctors, and that it<br />
must be reported to the authorities<br />
immediately. Such a thing is being<br />
done abroad and needs to be<br />
implemented in our country so that<br />
healthcare delivery is done in a safe<br />
and stress-free environment.<br />
May 2019 / FUTURE MEDICINE / 51
esearch snippets<br />
Removal of damaged mitochondria<br />
<strong>may</strong> stop inflammatory diseases<br />
Elsa Sanchez-Lopez et al have<br />
discovered that eliminating damaged<br />
mitochondria before they trigger<br />
excessive inflammation <strong>may</strong> help<br />
alleviate chronic inflammatory diseases.<br />
NLRP3 inflammasome- a multiprotein<br />
oligomer is responsible for the activation<br />
of inflammatory responses in the body.<br />
However, during mitochondrial damage<br />
due to stress or bacterial toxins NLRP3<br />
can remain ‘switched on’ leading to<br />
excess inflammation. Researchers<br />
have found that NLRP3 is deactivated<br />
when damaged mitochondria are<br />
eliminated during cellular cycling<br />
called mitophagy. Scientists showed<br />
that mitophagy can be induced by<br />
inhibiting the uptake of the nutrient<br />
choline by mitochondria. Choline is<br />
taken up through specific transporters<br />
and metabolized by enzyme choline<br />
kinase (ChoK). Decreasing the uptake of<br />
choline altered the mitochondrial lipid<br />
pro<strong>file</strong>, decreasing the ATP synthesis in<br />
mitochondria. This activates the energy<br />
sensor AMP-activated protein kinase<br />
(AMPK) which stimulates mitophagy.<br />
Choline uptake by cells was prevented<br />
by inhibiting choline transporter CTL1 or<br />
choline phosphorylation by using ChoK<br />
inhibitors. This attenuated the NLRP3<br />
inflammasome activation and IL-1β and<br />
IL-18 cytokine production in stimulated<br />
macrophages. Researchers also showed<br />
that on treating a mouse model of<br />
Muckle-Wells syndrome with ChoK<br />
inhibitors reversed the inflammation.<br />
The in vivo study<br />
in mice showed that treatment with<br />
ChoK inhibitors prevented acute<br />
inflammation caused by uric acid<br />
accumulation (seen in gout)<br />
and a bacterial toxin. ChoK<br />
inhibitor treatment also<br />
reversed chronic<br />
inflammation<br />
associated with a<br />
genetic disease<br />
called Muckle-<br />
Well Syndrome<br />
in mice which<br />
is caused by<br />
mutations in<br />
NLRP3 genes.<br />
Source: Cell Metabolism<br />
April 11, 2019 DOI: https://doi.<br />
org/10.1016/j.cmet.2019.03.011<br />
https://www.cell.com/cell-<br />
metabolism/fulltext/S1550-4131(19)30139-<br />
1#%20<br />
Faecal microbiota transfer could<br />
improve autism symptoms<br />
Dae-Wook Kang et al have<br />
demonstrated longterm<br />
beneficial effects of<br />
microbiota transfer therapy<br />
(MTT) in children with autism<br />
spectrum disorders (ASD). The<br />
researchers had previously<br />
conducted an open-label trial<br />
of microbiota transfer<br />
therapy in a cohort of<br />
18 children in 2017.<br />
The study involved 10<br />
weeks of treatment,<br />
including pre-treatment<br />
with vancomycin, a<br />
bowel cleanses, a<br />
stomach acid suppressant, and fecal<br />
microbiota transfer daily for seven to<br />
eight weeks. Two years since the study,<br />
a follow up conducted by researchers<br />
revealed significant improvements in<br />
gastrointestinal and autism-related<br />
symptoms in accordance with an<br />
increase in gut microbiota. Scientists<br />
reported a 45% decrease in ASD<br />
symptoms compared to baseline. At the<br />
start of the study, 83% of participants<br />
were rated to have severe autism. At the<br />
end of the study, only 17% were severe,<br />
39% were found to have moderate,<br />
and 44% were below the cut-off for<br />
moderate ASD. The findings showed<br />
52 / FUTURE MEDICINE / May 2019
Oxytocin could help treat alcohol dependence<br />
Brendan J. Tunstall et al demonstrated<br />
that administration of neuropeptide<br />
oxytocin <strong>may</strong> help normalize the alcohol<br />
use disorder and thereby help reduce<br />
alcohol addiction. The research was<br />
performed using alcohol-dependent<br />
rat models. The study demonstrated<br />
that oxytocin administered systemically,<br />
intranasally or into the brain blocked<br />
excess drinking in alcohol-dependent<br />
rats. Researchers found that the effect<br />
was specific to enhanced alcohol<br />
drinking problem in alcohol-dependent<br />
models as the procedure did not affect<br />
other normal behaviours or alcohol<br />
drinking in the alcohol-independent<br />
cohort. The researchers also found that<br />
the oxytocin blocked neurotransmitter<br />
gamma-aminobutyric acid (GABA)<br />
signalling in the central nucleus of the<br />
amygdala (CeA). CeA is a key brain<br />
region in the network affected by<br />
alcohol dependence. The study provides<br />
evidence suggesting that oxytocin likely<br />
blocks enhanced drinking by altering CeA<br />
GABA transmission. The findings provide<br />
evidence showing that aberrations in the<br />
oxytocin signalling <strong>may</strong> underlie alcohol<br />
use disorder which on targeting might<br />
prove a promising therapy in people who<br />
misuse alcohol.<br />
Source: PLOS Biology April 16, 2019 https://doi.<br />
org/10.1371/journal.pbio.2006421<br />
persistence of change in gut microbiota<br />
involving an increase in overall diversity<br />
and abundance of beneficial microbes<br />
including Bifidobacteria and Prevotella.<br />
The results encourage intensive MTT<br />
intervention as a promising therapy for<br />
treating children with ASD who have GI<br />
problems. The researchers recommend<br />
in conducting future research involving<br />
larger cohort trials.<br />
Source: Scientific Reports April 9, 2019 volume 9,<br />
Article number: 5821 (2019) https://www.nature.<br />
com/articles/s41598-019-42183-0<br />
Adiponectin plays key<br />
in male bias of liver<br />
cancer<br />
Elisa Manieri et al discovered that a<br />
decrease in a hormone secreted by<br />
fat cells is responsible for an increased<br />
risk of liver cancer in males. The<br />
levels of hormone adiponectin<br />
decrease in males during<br />
puberty which make them<br />
prone to a higher incidence<br />
of hepatocellular carcinoma<br />
(HCC) than women. HCC<br />
risk is also higher among<br />
obese individuals during<br />
which the body secretes<br />
low adiponectin levels.<br />
Researchers found that<br />
adiponectin protects against<br />
liver cancer development<br />
through the activation of AMPactivated<br />
protein kinase (AMPK)<br />
and p38α. The study showed that<br />
testosterone in males activates JNK<br />
protein (c-Jun N-terminal kinases) in<br />
human and mice adipocytes. JNK protein<br />
mediates inhibition of adiponectin<br />
which results in their lower secretion.<br />
The research also showed that genetic<br />
deletion of JNK1 in mouse adipose<br />
tissue resulted in higher adiponectin<br />
levels and protection against HCC.<br />
Quantification of circulating adiponectin<br />
in mice models detected more than<br />
twice the level in females than in males.<br />
This correlated with the vigorous growth<br />
of subcutaneously implanted mouse<br />
HCC-derived tumour cells in males than<br />
in females. Gender disparity in HCC was<br />
confirmed by subcutaneously injecting<br />
colon adenocarcinoma-derived tumour<br />
cells or melanoma-derived tumour in<br />
male and female mice models which<br />
showed no difference in growth patterns.<br />
The study unravels the relation between<br />
sex hormones and adipocytes signalling<br />
clarifying the disparity in liver cancer<br />
development.<br />
Source: Journal of Experimental Medicine April<br />
3, 2019 DOI: 10.1084/jem.20181288 http://<br />
jem.rupress.org/content/early/2019/04/02/<br />
jem.20181288<br />
Alcohol-induced brain<br />
damage continues<br />
after cessation<br />
Silvia De Santis et al show evidence<br />
in the progression of damage within<br />
the white matter of the brain during<br />
the initial period of alcohol cessation.<br />
The researchers used Diffusion<br />
Tensor Imaging (DTI), a magnetic<br />
resonance imaging-based<br />
technique capable of detecting<br />
the anisotropy of the brain’s<br />
white matter tracts. The study<br />
revealed that the damage<br />
in the brain could continue<br />
up to two to six weeks of<br />
abstinence from alcohol. The<br />
study involved 91 volunteers<br />
of average 46 years of age<br />
who had alcohol use disorder<br />
(AUD) and were interned for<br />
rehabilitation. A control group<br />
involving 36 men without alcohol<br />
problems with an average age<br />
May 2019 / FUTURE MEDICINE / 53
of 41 years was also involved in the<br />
study. The findings showed comparable<br />
alterations in the white matter with<br />
intense preferential involvement of<br />
corpus callosum and fimbria which are<br />
connected with memory and decision<br />
making. The results were compared<br />
in parallel with rat models showing<br />
preference to alcohol consumption. 27<br />
male rats showing AUD were compared<br />
against 9 male control rats. The research<br />
reveals that the damage progression<br />
can still continue in the brain during<br />
initial abstinence from severe alcohol<br />
consumption.<br />
Source: JAMA Psychiatry April 3, 2019<br />
doi:10.1001/jamapsychiatry.2019.0318 https://<br />
jamanetwork.com/journals/jamapsychiatry/articleabstract/2729425<br />
Electrostimulation<br />
can improve working<br />
memory in elderly<br />
Robert M. G. Reinhart et al<br />
demonstrated that electrostimulation<br />
can improve the working memory of<br />
older adults by temporarily reversing<br />
the age-related cognitive decline. The<br />
study involved 42 people aged 20–29<br />
years and 42 people aged 60–76 years<br />
and was assessed in a working memory<br />
task. The study showed that the core<br />
feature of cognitive decline was caused<br />
due to a disconnection between the two<br />
brain networks of the frontotemporal<br />
cortex. The researchers subjected the<br />
older participants to 25 minutes of noninvasive<br />
electrical stimulation across the<br />
brain using electrodes with frequency<br />
personalized to their individual brain<br />
circuits. After the stimulation the cohort<br />
showed a preferential increase in neural<br />
synchronization patterns between the<br />
frontotemporal regions of the brain. All<br />
participants were subjected to test their<br />
working memory. The results before<br />
and after stimulation were compared<br />
between the two cohorts. The findings<br />
revealed that the older participants<br />
showed a rapid improvement in working<br />
memory that lasted for 50 minutes post<br />
stimulation. The findings unveil a new<br />
approach towards non-pharmacological<br />
intervention in improving cognitive<br />
decline by targeting aspects of agerelated<br />
cognitive impairment.<br />
Source: Nature Neuroscience April 8,2019 https://<br />
www.nature.com/articles/s41593-019-0371-x<br />
—Compiled by Divya Choyikutty<br />
Scientists `print’ 3D heart using patient’s own cells<br />
Nadav Noor et al have successfully<br />
printed the first 3D vascularised<br />
engineered heart using a patient’s<br />
own cells and biological materials.<br />
The researchers utilized biopsy of<br />
fatty tissue from the patient<br />
for the study. The cells were<br />
then reprogrammed to<br />
become pluripotent stem<br />
cells that differentiated<br />
into cardiomyocytes and<br />
endothelial cells. The<br />
extracellular matrix (ECM)<br />
which is a three-dimensional<br />
network of extracellular<br />
macromolecules such as collagen<br />
and glycoproteins were processed into<br />
personalized hydrogel that served as<br />
the printing ink. The two cell types were<br />
separately combined with the hydrogels<br />
to form the bio ink for parenchymal<br />
cardiac tissue and blood vessels. Blood<br />
vessel architecture was developed by<br />
mathematical modelling of oxygen<br />
transfer. The researchers demonstrated<br />
the ability to print functional, patient<br />
specific, immune-compatible cardiac<br />
patches with blood vessels forming the<br />
entire heart. The study thus revealed<br />
the first successful approach to 3D-print<br />
personalized, thick, vascularised and<br />
perfusable cardiac tissues which <strong>may</strong><br />
be developed for drug screening and<br />
patient specific requirements in the<br />
future.<br />
Source: Advanced Science 15 April 2019 https://<br />
doi.org/10.1002/advs.201900344<br />
54 / FUTURE MEDICINE / May 2019
Ultrasound<br />
All-in-one imaging.<br />
Complete solution.<br />
Breast cancer is the most common cancer among women, early detection is key<br />
to saving lives. Introducing eL18-4, a single probe solution for breast assessment.<br />
It allows clinicians to effectively assess, monitor and treat breasts diseases;<br />
PureWave crystal technology on<br />
eL18-4 enables transducer to achieve<br />
superb clinical performance across<br />
various patients including technically<br />
difficult patients.<br />
Complete Elastography solutions for<br />
rapid assessment of whole array of<br />
breasts lesions with enhanced<br />
confidence.<br />
Anatomical intelligence facilitates<br />
breast exam while preserving superb<br />
image quality.<br />
Assess<br />
Monitor<br />
Treat<br />
New precision biopsy capabilities<br />
reduce needle blind zone and<br />
ensures enhanced display of needle<br />
reflections.<br />
For more information, please visit:<br />
www.philips.co.in/healthcare or call 1800-419-8844 (Toll Free)
hospital news<br />
Everstone to buy controlling<br />
stake in Sahyadri Hospitals<br />
Everstone, an investment group, has<br />
agreed to buy a 51% stake in Sahyadri<br />
Hospitals Ltd (SHL).<br />
Pune-based Sahyadri Hospitals is the<br />
largest hospital chain in Maharashtra,<br />
founded in 1994. Currently, it operates<br />
five tertiary care and three secondary<br />
care hospitals with 750 beds across<br />
Pune, Nashik and Karad. Sahyadri has<br />
more than 1,000 clinicians and 2,300<br />
supporting staff, reports said.<br />
The transaction is expected to<br />
help Sahyadri further strengthen its<br />
position as the leading healthcare<br />
chain in Maharashtra and increase its<br />
bed-count significantly in the next five<br />
years.<br />
For Everstone, the deal will increase<br />
its exposure to India’s healthcare sector<br />
where it is already present in numerous<br />
segments such as pharmaceuticals and<br />
diagnostics.<br />
LVPEI completes<br />
100 transplants<br />
in rural India<br />
V Prasad Eye Institute (LVPEI)<br />
L performed its 100th corneal<br />
transplant in a rural eye care centre at<br />
Mudhole secondary eye centre.<br />
Started in March 2016 with a<br />
corneal transplant at the Institute’s<br />
Dhulipalla secondary eye centre in<br />
Guntur District, experts from LVPEI<br />
have collectively performed a hundred<br />
corneal transplants in a span of just<br />
three years across the secondary<br />
centre networks in Mudhole (Nirmal<br />
District), Dhulipalla (Guntur District),<br />
Venkatachalam (Nellore District) and<br />
Paloncha (Bhadradri Kothagudem<br />
District).<br />
“This achievement is an example<br />
of a road never travelled,” stated Dr<br />
Gullapalli N Rao, founder and chair, L V<br />
Prasad Eye Institute in a statement.<br />
India is home to half of the world’s<br />
blind population. Out of the 15 million<br />
blind people in the country, 6.8 million<br />
are victims of corneal blindness. This<br />
number is expected to rise to 10.6<br />
million by 2020.<br />
Healthcare sector outlook stable: ICRA<br />
India’s healthcare sector outlook remains<br />
stable despite regulatory headwinds, says<br />
ICRA, an Indian credit rating agency.<br />
The hospital sector’s performance has<br />
been falling in the last two years due to<br />
several regulatory restrictions, including<br />
demonetisation, the cap on prices of<br />
stents and knee implants by the National<br />
Pharmaceutical Pricing Authority (NPPA),<br />
stiff regulatory action by certain states<br />
and the implementation of the Goods and<br />
Services Tax (GST).<br />
In the latest order, issued on March 8,<br />
2019, the NPPA put a cap of 30 per cent<br />
on the trade margin earned on 390 drugs<br />
that are primarily used for treatment of<br />
cancer. The price cap led to a reduction of<br />
up to 87 per cent in the prices of the anticancer<br />
drugs. The current order by NPPA<br />
adds on to the list of products already<br />
placed under price restrictions. Most of<br />
the cancer-related drugs are high-value<br />
medicines and are sold directly through<br />
the hospital chains, oncology being one<br />
of the highest-value-added specialty for<br />
hospitals due to the increasing incidence<br />
of cancer-related ailments. Nonetheless,<br />
several oncology drugs are still out of the<br />
purview of the price cap.<br />
“The regulatory environment continues<br />
to be the overarching challenge for the<br />
hospital sector and the recent NPPA order<br />
reinforces this view. The wide-ranging<br />
regulatory restrictions from multiple<br />
authorities have suppressed the margins<br />
of the players,’’ said Shubham Jain, group<br />
head and vice president – corporate sector<br />
ratings, ICRA, in a statement.<br />
However, hospitals’ decision on<br />
repricing of service/product component of<br />
packages and usage of different drugs is<br />
expected to correct the lopsided pharma<br />
component margins and partly negate<br />
the impact of the current NPPA order, he<br />
noted.<br />
56 / FUTURE MEDICINE / May 2019
from the industry<br />
“OPHTHALMOLOGISTS ARE<br />
MOST OVERSTRETCHED IN<br />
INDIA, SO OUR AIM IS TO HELP<br />
THEM REMAIN STRAIN-FREE”<br />
With 15 million blinds, India is home for 50% of<br />
the world’s total blind population, according to<br />
a 2016 study by the World Health Organisation.<br />
While the status hasn’t changed much in the last two years,<br />
another most worrying fact is that the country is also one<br />
of the geographies in the world where the number of<br />
ophthalmologists in proportion to its total population is the<br />
lowest. And thus, it wasn’t a surprise that why the country was<br />
also infamous for the highest number of medical negligence<br />
in the area of eye care until recently. While the cases of<br />
negligence have significantly come down in the last few years,<br />
the credit to a great extent should go to the technology players<br />
who changed the scenario by bringing the much-needed<br />
sophistication into this field of care. Sandeep Bothra, Country<br />
Business Head- Surgical, Alcon Laboratories India, says that<br />
most of the recent technologies, those were introduced in<br />
the area of eye care in India, have visibly improved the way<br />
the diagnosis and procedures are done in the eye care setups<br />
and the emerging technologies will make the visualisation and<br />
diagnosis more easier and accurate, in an interview with editor<br />
CH Unnikrishnan. Edited excerpts:<br />
Eye care scenario in India has been quite challenging<br />
because of several factors including a shortage of<br />
ophthalmologists, lack of better training and infrastructure<br />
and accessibility issues with best technologies. Alcon has<br />
been in India for some time now and could you tell us what<br />
has been your experience so far and do you think the<br />
market has improved in terms of quality of care?<br />
Alcon as a global leader in eye care, we always try to<br />
discover new ways to enhance sight and improve patient lives.<br />
As we have been doing this successfully in this market through<br />
innovative products, partnerships with eye care professionals<br />
and programs that create greater access to quality eye care.<br />
We identify and develop technologies that deliver better visual<br />
outcomes and address unmet patient needs, continuously<br />
improving the options that exist today. Since our research<br />
team observes surgeries and visits clinics to gain real-world<br />
insights, we also regularly meet with<br />
eye care professionals to get their<br />
feedback on our products and their<br />
future needs. So, I can confidently say<br />
that the technology and training have<br />
significantly improved the situation in<br />
the Indian eye care. Also, the customers,<br />
as well as patients, have become quite<br />
demanding as they are already aware<br />
of the latest products and procedures.<br />
It is also evident from the change that<br />
the cases of medical negligence have<br />
come down drastically in recent time.<br />
And I am proud to say that our products<br />
touch the lives of millions of people<br />
each year living with conditions like<br />
cataracts, glaucoma, retinal diseases,<br />
and refractive errors. Since we are the<br />
only company that offers the complete<br />
line of ophthalmic surgical devices, as<br />
well as a differentiated contact lens and<br />
lens care portfolio, this also gives us a<br />
premium and most trusted position in<br />
the market.<br />
Alcon, through its social<br />
responsibility and advocacy efforts,<br />
also help to create sustainable access<br />
to eye care for patients around the<br />
world, thereby reducing the incidence<br />
of preventable blindness and visual<br />
impairment. By investing in professional<br />
education, the group help to advance<br />
the eye care industry and ultimately<br />
create better outcomes for patients and<br />
consumers.<br />
How do you see the pricing scenario<br />
here as the government is slowly<br />
58 / FUTURE MEDICINE / May 2019
vast country with varied needs and priorities depending<br />
on the pro<strong>file</strong>s of different regions, optimising the<br />
market opportunities will depend on the adaptability<br />
of the players. Still, I feel the country offers ample<br />
opportunities for both national and international<br />
companies.<br />
As you know, India is also a very competitive as<br />
well as value conscious market with so many players,<br />
including foreign and local brands in the ophthalmic<br />
segment. So, what made you the largest and how do<br />
you maintain the leadership?<br />
The key factor that made us the market leader is<br />
the unparalleled reach that we have in this market. We<br />
are present in every corner of the market, spanning<br />
from one end to the other in the length and breadth<br />
of the country. In addition, we have the entire portfolio<br />
of products catering to every disease and age groups.<br />
The other important factor is the quality and innovation<br />
that we maintain in every class of product knowing our<br />
partners’ needs and helping them consistently with the<br />
latest technologies, enhancing their choice of practice.<br />
That’s why the surgeons love to work with us.<br />
bringing cost regulation on most<br />
medical devices, implants and<br />
health equipment?<br />
A couple of thoughts on this.<br />
No doubt, the intention of the<br />
government is noble as it wants to<br />
expand the access of healthcare<br />
to every stratum of society. But I<br />
would say, the opportunity in India<br />
for every segment is big enough<br />
for everybody. In general, I think<br />
the changes in market dynamics<br />
will ultimately depend on how<br />
these are rolled out and how agile<br />
are the companies to adapt to the<br />
changing requirements. India as a<br />
The key factor<br />
that made us the<br />
market leader is<br />
the unparalleled<br />
reach that we have<br />
in this market. We<br />
are present in every<br />
corner of the market,<br />
spanning from one<br />
end to the other in the<br />
length and breadth of<br />
the country.<br />
What are the key challenges in this market?<br />
In the current context, one of the challenges is how<br />
do you ensure that our products and services are<br />
available in the same quality across the country. For<br />
example, if we want to sell or service our products in<br />
Imphal or Mandya, Srinagar or Lakshadweep, I want<br />
to ensure the same quality of service that I provide in<br />
Mumbai or Delhi. But, managing the logistics of that is<br />
a big challenge in such a vast country. Just to explain<br />
the technical aspect of it, I would take the example<br />
of a cataract surgery, where there are two important<br />
equipment required—the main equipment and the<br />
library of implants. In this case, we have to reach the<br />
entire library even if the surgeon uses just one particular<br />
implant, which is decided at the surgery table. So, we<br />
need to make the stock available so that the doctor can<br />
do the surgery without compromising the quality. And<br />
this is about every geography and climatic conditions.<br />
Similarly, the doctors, though fundamentally they are<br />
the same everywhere, in India often need to balance<br />
the quality of work as well as the value proposition<br />
as the majority of the patients here still pay out of<br />
pocket. While the lack of patient awareness is a<br />
problem, the work pressure that is often faced by the<br />
overstretched surgeons in this branch of medicine is<br />
another big challenge. In India, there are only 12 to 13<br />
ophthalmologists for a million patients, which is much<br />
below the global average. So, we aim to overcome<br />
these challenges, both at the patient side as well as the<br />
clinical side using more innovative technologies.<br />
May 2019 / FUTURE MEDICINE / 59
column<br />
trialomics<br />
Speedy approval vs<br />
safety?<br />
Unless Indian regulators develop in-house expertise, the<br />
fast-track approval for investigational drugs could put<br />
clinical trial participants at high risk<br />
DR ARUN BHATT<br />
Writer is a consultant<br />
on clinical research &<br />
development from<br />
Mumbai.<br />
arun_dbhatt@hotmail.com<br />
Recently released new Indian drug<br />
rules have been hailed as allowing<br />
fast and time-bound approval within<br />
30 calendar days for clinical trials of “Indian<br />
new drugs”. This step can improve “ease of<br />
business”, support “Make in India”, and will<br />
encourage innovation in drug discovery. But<br />
this is a major shift from the uncertain and<br />
long regulatory approval process of the last<br />
few years. Hence, the concern is how Indian<br />
health authorities (HA) plan to implement<br />
this. Will the expedited approval be at the<br />
cost of ensuring protection for Indian clinical<br />
trial participants?<br />
The rules define an “Indian new drug”<br />
as 1) a drug discovered in India, or (2)<br />
its research and development (R&D),<br />
manufacture, and marketing are done<br />
in India. Current Indian cost of R&D,<br />
extrapolated from Dr Mashelkar<br />
committee’s 1999 estimate of Rs 150 crores,<br />
would be Rs 500 crores. In contrast, the<br />
US R&D cost estimate of $ 2.6 billion (Tufts<br />
Center 2016) or Rs 19,500 crores is 39<br />
times higher! Considering the tremendous<br />
cost advantage, quicker approval and<br />
faster completion of clinical trials, western<br />
companies would find it commercially<br />
attractive to develop a new drug in India<br />
in collaboration with an Indian partner.<br />
Such collaborations will boost Indian drug<br />
research capabilities.<br />
New drug development begins with<br />
phase I trials. In this first-in-human (FIH) trial,<br />
serious adverse events (SAE) can jeopardize<br />
the safety of clinical trial participants. In<br />
2006, TGN1412 resulted in life-threatening<br />
multiorgan failure in all 6 healthy volunteers<br />
who received the first dose of the drug. In<br />
2016, BIA 10-2474 caused the death of a<br />
healthy volunteer and SAEs in 4 participants<br />
who received the drug. Hence, thorough riskbenefit<br />
assessment of an investigational new<br />
drug (IND) is essential before approving FIH<br />
clinical trial.<br />
US Food and Drug Administration (FDA)<br />
allows a pharma company to initiate<br />
phase I trial 30-days after receiving IND<br />
application. But FDA can place the trial<br />
on-hold if it finds that human subjects<br />
would be exposed to unreasonable and<br />
significant risk. FDA has an in-house team<br />
of pharmacologists, toxicologists and clinical<br />
pharmacologists to review IND application.<br />
Indian HA depends on IND committee for<br />
review and approval of the phase I trial.<br />
This committee, consisting of ICMR experts<br />
and other consultants, meets at intervals<br />
of 2-3 months to review pre-clinical data -<br />
animal pharmacology, pharmacokinetic,<br />
and toxicity - and decide whether the phase<br />
I trial should be approved. The committee<br />
<strong>may</strong> ask for additional pre-clinical data or<br />
modifications in the clinical trial protocol.<br />
For Indian R&D based pharma companies,<br />
which have complained about long and<br />
unpredictable IND review process, the new<br />
30-day clinical trial approval is a dream<br />
come true. However, unless Indian HA<br />
develop in-house expertise or establish a<br />
fast track IND committee process before the<br />
30-day approval rule and have well-defined<br />
post-approval oversight process and clinical<br />
hold rules, Indian clinical trial participants<br />
will be at high risk from investigational new<br />
drugs.<br />
60 / FUTURE MEDICINE / May 2019
NGM2<br />
NextGen Molecular Medicine<br />
NGM2 is a platform for clinical and molecular worlds to meet and<br />
create better healthcare systems in India<br />
A joint initiative of Institute for Applied Research<br />
and Innovation and Future Medicine to<br />
enable better clinical practices<br />
WE OFFER HANDS-ON<br />
TRAINING IN<br />
• NextGen technologies<br />
in clinical practice<br />
• Emerging technologies<br />
in diagnostics and research<br />
• Training in clinical research<br />
and biostatistics<br />
• Applied bioinformatics<br />
and clinical informatics<br />
• Clinical data analyticals<br />
Our workshops are<br />
currently being<br />
offered at<br />
NITTE<br />
Medical University,<br />
Mangalore<br />
and<br />
InARI,<br />
Bangalore<br />
WHO<br />
CAN<br />
ATTEND?<br />
Doctors, Molecular scientists (Ph.D and post-docs),<br />
Students (MBBS, BSc, MSc, BTech and MTech)<br />
Special focus on medical students to learn new<br />
emerging technologies for improved clinical practices<br />
Please contact us @<br />
reach.inari@gmail.com / +91 9880568987<br />
in association with<br />
NextGen Molecular M<br />
(NGM2)<br />
- A workshop seri<br />
NGM2 is a platform for clinical an
devices&gadgets<br />
Breakthrough status to<br />
preeclampsia device<br />
Targeted Apheresis<br />
Column for Preeclampsia<br />
(TAC-PE) has been<br />
granted a Breakthrough<br />
Device Designation by the<br />
USFDA, Advanced Prenatal<br />
Therapeutics announced.<br />
The device specifically<br />
removes disease-causing<br />
factors such as sFlt-1 from the<br />
mother’s blood. It is intended<br />
to treat preeclampsia, a<br />
leading cause of prematurity<br />
and maternal/foetal death,<br />
and holds the potential to<br />
substantially reduce<br />
mortality, incidence or<br />
severity of preterm birth, and<br />
the use of neonatal intensive<br />
care.<br />
The company is now<br />
preparing for initial clinical<br />
studies using the device.<br />
The FDA grants<br />
Breakthrough Device<br />
Designation to devices<br />
that provide more effective<br />
treatment of life-threatening<br />
or irreversibly debilitating<br />
diseases. Devices must also<br />
represent a breakthrough<br />
technology, treat a disease<br />
where no approved<br />
alternatives exist, offer<br />
significant advantages<br />
over existing approved<br />
alternatives, or otherwise<br />
be in the best interest of<br />
patients.<br />
501k clearance for pain drug delivery device<br />
Avanos Medical has<br />
received US FDA 501(k)<br />
clearance for its ON-Q with<br />
bolus pump.<br />
The new ON-Q with<br />
bolus design incorporates<br />
improvements that make it<br />
simpler for providers and<br />
patients to use while reducing<br />
postoperative opioid use to<br />
achieve pain management.<br />
The new ON-Q bolus<br />
provides customised control<br />
for patients recovering<br />
from post-surgical pain by<br />
delivering continuous, nonopioid<br />
medication to the<br />
surgical site or peripheral<br />
nerves for up to five days.<br />
The bolus pump is<br />
ergonomically designed to fit<br />
comfortably in the patient’s<br />
hand with an easy to remove<br />
the priming tab and large<br />
level indicator markings. The<br />
device can also be used for<br />
incisional applications.<br />
Studies have shown that<br />
using bolus rates as low<br />
as 3 ml in addition to the<br />
basal rate can reduce the<br />
total amount of anaesthetic<br />
needed for pain relief. ON-Q<br />
is indicated to significantly<br />
reduce opioid use for surgical<br />
patients and provide better<br />
pain relief than opioids alone,<br />
helping patients to get back<br />
to normal faster after surgery.<br />
62 / FUTURE MEDICINE / May 2019
located on the top face and<br />
both sides of the monitor for<br />
easy access and comfort.<br />
Omron developed<br />
Complete in partnership with<br />
AliveCor, the market leader<br />
in FDA-cleared personal EKG<br />
technology. Complete uses<br />
the advanced new algorithm<br />
designed by AliveCor for<br />
improved detection of the<br />
possibility of Afib along with<br />
trusted medical-grade blood<br />
pressure measurement from<br />
Omron.<br />
Complete is the recipient<br />
of the iF Design Award 2019,<br />
presented by iF International<br />
Forum Design GmbH.<br />
US FDA approves<br />
novel portable<br />
gas exchange<br />
monitor<br />
T<br />
he US FDA has approved<br />
the Gas Exchange Monitor,<br />
a portable respiratory<br />
monitoring device.<br />
The device provides realtime,<br />
clinically actionable<br />
data measuring pulmonary<br />
gas exchange in a wide<br />
Contec<br />
Americas<br />
launches medical<br />
monitors<br />
Contec Americas Inc has<br />
developed a new line<br />
of medical-grade monitors<br />
to meet the stringent<br />
image quality and safety<br />
requirements of clinical<br />
applications. The initial release<br />
of seven displays features<br />
DICOM Part 14 image quality<br />
and IEC 60601 compliance<br />
with medical safety and<br />
performance standards.<br />
The monitors are designed<br />
for original equipment<br />
manufacturers (OEMs) looking<br />
for versatile, long life solutions<br />
suited for a wide variety<br />
of hospital and laboratory<br />
applications.<br />
Contec’s new clinical<br />
displays range in size from<br />
15” to 27” and include 5-wire<br />
resistive touch and projected<br />
capacitive touch (PCAP)<br />
options.<br />
The Legacy group features<br />
a 4:3 aspect ratio.<br />
Contec’s Modern<br />
line boasts a sleek,<br />
contemporary<br />
look, 16:9<br />
widescreen<br />
aspect ratio, high<br />
brightness up to 350<br />
nits, wide viewing angles<br />
up to 178 degrees and trueflat<br />
front, IP65 rating for easy<br />
cleaning and sanitation.<br />
Contec’s new clinical<br />
display line is part of a series<br />
of new products which will be<br />
introduced in 2019.<br />
Omron<br />
introduces home<br />
BP monitor with<br />
EKG capability<br />
Omron Healthcare has<br />
secured USFDA clearance<br />
on its new Complete, the<br />
first blood pressure monitor<br />
with EKG capability in a single<br />
device.<br />
Complete is an upper<br />
arm blood pressure monitor<br />
that allows users to<br />
simultaneously monitor EKG<br />
and blood pressure readings<br />
at home. EKG readings can<br />
be measured by touching<br />
electrodes conveniently<br />
Tack endovascular system for<br />
PAD gets US FDA approval<br />
Intact Vascular, Inc has received<br />
the US FDA approval for the<br />
Tack Endovascular System (6F), a<br />
dissection repair device implanted<br />
post-angioplasty in patients with<br />
the peripheral arterial disease<br />
(PAD).<br />
The approval was based on<br />
data from Intact Vascular’s Tack<br />
Optimized Balloon Angioplasty<br />
II (TOBA II) pivotal trial, which<br />
demonstrated the safety and<br />
effectiveness of the Tack implant<br />
to resolve dissections following<br />
angioplasty.<br />
The inflation of an angioplasty<br />
balloon and resulting mechanical<br />
stress inherently injures vessels<br />
and creates dissections. If left<br />
untreated, dissections can<br />
compromise clinical outcomes,<br />
resulting in acute thrombosis and<br />
arterial occlusions, leading to lower<br />
long-term patency rates and repeat<br />
procedures.<br />
The TOBA II pivotal trial,<br />
notably the first peripheral<br />
vascular study to enroll<br />
patients with 100%<br />
dissected vessels, met all<br />
primary endpoints with<br />
92% of dissections completely<br />
resolved following treatment, the<br />
company reported.<br />
May 2019 / FUTURE MEDICINE / 63
variety of respiratory disease,<br />
manufactured and marketed<br />
by MediPines.<br />
The Novel physiologic<br />
results on world elite<br />
freedivers’ using the noninvasive<br />
gas exchange<br />
monitor were presented at<br />
the Experimental Biology<br />
2019 conference. The<br />
new device makes it now<br />
possible for a non-invasive<br />
portable measurement that<br />
can support evaluation and<br />
trending of pulmonary gas<br />
exchange status of a patient<br />
in a point of care setting.<br />
Freedivers place<br />
themselves in extreme<br />
conditions, which push the<br />
outer limits of human lung<br />
physiology and can lead<br />
to a high oxygen deficit.<br />
The device provided rapid<br />
feedback to the research<br />
team tasked with assessing<br />
the freedivers’ gas exchange<br />
status.<br />
Baxter launches<br />
haemostatic<br />
product<br />
Floseal Hemostatic Matrix<br />
has been granted approval<br />
by the USFDA, Baxter<br />
International announced.<br />
Floseal has 20 per cent<br />
fewer components and steps<br />
to prepare, making it easier<br />
and faster for operating room<br />
(OR) nurses to get Floseal<br />
in the hands of surgeons to<br />
help stop bleeding during<br />
procedures.<br />
The device has been<br />
proven to perform quickly and<br />
consistently across a range of<br />
bleeds in surgical procedures.<br />
A 13cm Malleable Applicator<br />
is included with every kit that<br />
allows surgeons to maneuver<br />
the product into the proper<br />
position.<br />
In this latest design, the<br />
diluent ampoule has been<br />
replaced by pre-filling the<br />
existing mixing syringe so<br />
that Floseal can be prepared<br />
more quickly than the current<br />
configuration.<br />
Both active and passive<br />
adjunctive haemostatic<br />
Cryotherapy device to treat<br />
heavy menstrual bleeding<br />
Cerene cryotherapy device<br />
has been granted approval<br />
by US FDA as a new approach<br />
to treating heavy menstrual<br />
bleeding.<br />
The device uses cryotherapy<br />
to freeze the endometrial<br />
lining of the uterus to reduce<br />
future menstrual bleeding in<br />
premenopausal women who<br />
are not planning to become<br />
pregnant.<br />
This procedure does not<br />
require general anaesthesia. It<br />
can, therefore, be performed<br />
in the gynaecologist’s office<br />
in contrast, heat-based<br />
endometrial ablation devices<br />
which are more often<br />
performed with general<br />
anaesthesia in hospitals.<br />
CLARITY, the pivotal study<br />
supporting the safety and<br />
effectiveness of the Cerene<br />
device and its FDA approval<br />
included treatment of 242<br />
subjects.<br />
At 12 months, the observed<br />
reduction in menstrual bleeding<br />
exceeded treatment goals.<br />
64 / FUTURE MEDICINE / May 2019
agents are available to help<br />
control bleeding in surgical<br />
procedures when ligature or<br />
conventional methods are<br />
ineffective or impractical.<br />
US FDA approves<br />
Duaklir inhaler<br />
for COPD<br />
The US FDA has approved<br />
Duaklir Pressair for the<br />
maintenance treatment of<br />
chronic obstructive pulmonary<br />
disease (COPD).<br />
Duaklir Pressair combines<br />
aclidinium bromide, a longacting<br />
muscarinic antagonist<br />
(LAMA), and<br />
formoterol fumarate, a<br />
long-acting beta2 -adrenergic<br />
agonist (LABA). It is intended<br />
for twice-daily use with the<br />
breath-actuated Pressair<br />
inhaler.<br />
The approval was<br />
supported by data from the<br />
phase 3 ACLIFORM, AUGMENT,<br />
and AMPLIFY studies which<br />
included patients with<br />
moderate to very severe<br />
COPD.<br />
Results showed that<br />
treatment with Duaklir<br />
Pressair led to a statistically<br />
significant increase in mean<br />
change from baseline in<br />
trough FEV1 and change from<br />
baseline in 1-hour post-dose<br />
FEV1 at week 24 relative to<br />
formoterol fumarate 12mcg<br />
and aclidinium 400mcg,<br />
respectively.<br />
Circassia who is planning<br />
to launch Duaklir in the US in<br />
the second half of 2019 via its<br />
dedicated COPD sales force.<br />
Medtronic<br />
launches spinal<br />
device for pain<br />
management<br />
Medtronic has launched<br />
Intellis platform for the<br />
management of certain types<br />
of chronic intractable pain.<br />
EU nod for endoscopic ablation system<br />
HeartLight X3 Endoscopic<br />
Ablation System has<br />
received European CE<br />
Mark approval for ablation<br />
treatment for atrial<br />
fibrillation.<br />
HeartLight X3<br />
Endoscopic Ablation System<br />
is a third-generation<br />
technology building upon<br />
the advanced features of<br />
the HeartLight Endoscopic<br />
Ablation System, which<br />
performs pulmonary vein<br />
isolation (PVI) using laser<br />
The platform was designed<br />
to overcome limitations with<br />
current spinal cord stimulation<br />
(SCS) systems, such as battery<br />
performance, and can power<br />
the Evolve workflow, which<br />
standardises guidance and<br />
balances high-dose (HD)<br />
and low-dose (LD) therapy<br />
settings.<br />
It can record and track<br />
patient activity 24/7 and is<br />
managed on the Samsung<br />
Galaxy Tab S2 tablet interface,<br />
enabling physicians to address<br />
the subjective and personal<br />
nature of chronic pain by<br />
monitoring progress and<br />
making modifications to better<br />
suit their patients’ therapy<br />
needs.<br />
Intellis platform can help<br />
optimize treatment and<br />
improve patient-physician<br />
energy to create lines<br />
of scar tissue to block<br />
the abnormal electrical<br />
pathways that cause AFib.<br />
Using direct tissue<br />
visualization, titratable<br />
laser energy, and compliant<br />
balloon technology, the<br />
HeartLight X3 System’s<br />
unique RAPID mode<br />
leverages a precise motor<br />
control system that enables<br />
uninterrupted, high-speed,<br />
circumferential lesion<br />
creation under the direct<br />
communication by tracking<br />
and sharing daily activities,<br />
body positions and therapy<br />
usage and by giving physicians<br />
an objective look at mobility<br />
and progress.<br />
The device also addresses<br />
a common patient complaint:<br />
battery recharge issues.<br />
control of the physician<br />
resulting in consistently<br />
reduced procedure times.<br />
In the pivotal<br />
confirmatory evaluation of<br />
60 patients, the HeartLight<br />
X3 System consistently<br />
achieved very rapid PVI, in<br />
as few as three minutes for<br />
a single vein. The trial also<br />
found that the System has<br />
the potential to complete<br />
all required ablations in less<br />
than 20 minutes.<br />
With Medtronic’s proprietary<br />
Overdrive battery technology,<br />
the Intellis battery can be fully<br />
recharged from empty to full<br />
in approximately one hour and<br />
physicians can now estimate<br />
recharge intervals based on<br />
therapy settings.<br />
Additional advances in<br />
the device include secure<br />
wireless Samsung Galaxy Tab<br />
S2 programmers for physicians<br />
that enable faster delivery<br />
of evolving workflows and<br />
software upgrades.<br />
Medtronic neurostimulation<br />
therapy for chronic intractable<br />
pain uses a medical device<br />
placed under a patient’s<br />
skin to deliver mild electrical<br />
impulses through a lead<br />
implanted in the epidural<br />
space to block pain signals<br />
from going to the brain.<br />
May 2019 / FUTURE MEDICINE / 65
ADVERTORIAL<br />
EPIQ Elite ultrasound line<br />
with vascular imaging<br />
Royal Philips’ EPIQ Elite ultrasound<br />
system offers a range of diagnostic<br />
ultrasound solutions tailored to the needs<br />
of specific medical specialties including<br />
solution for vascular assessment and<br />
diagnosis.<br />
In Obstetrics & Gynecology EPIQ Elite<br />
delivers extraordinary image quality and<br />
lifelike 3D scans to provide advanced fetal<br />
assessment during all stages of pregnancy.<br />
The Philips ultimate ultrasound solution<br />
for vascular assessment provides superb<br />
insight into the progression of vascular<br />
disease. It brings 3D and 4D imaging to<br />
vascular ultrasound for the first time that<br />
effectively help analyze vascular disease<br />
allowing clinicians to see into a vessel to<br />
spot plaque and assess flow data over time.<br />
The EPIQ Elite for obstetrics and<br />
gynecology combines with innovative<br />
transducers and software to offer an<br />
integrated solution that enhances fetal<br />
assessment and meets the specific needs<br />
of obstetrics patients during the early<br />
stages of pregnancy.<br />
FEATURES<br />
EPIQ Elite combines new display<br />
technology, innovative transducers,<br />
advanced software and enhanced<br />
processing power.<br />
xMATRIX transducer: Philips’ innovative<br />
xMATRIX transducer can produce<br />
extraordinary 3D vascular images, allowing<br />
clinicians to easily see directly into a vessel<br />
to evaluate plaque spatial location and<br />
composition, as well as view 3D flow data<br />
to quickly assess stenotic conditions.<br />
‘xPlane’ imaging: proprietary to Philips<br />
– allows clinicians to acquire two planes<br />
simultaneously to improve accuracy of<br />
data collection, and reduce exam time by<br />
20%.<br />
Icon-driven visual workflow : makes 3D<br />
imaging easier, reducing the steps that the<br />
clinician needs to take from 10 to one.<br />
V9-2 transducer : The ergonomic,<br />
lightweight V9-2 transducer is the first<br />
high-frequency PureWave transducer<br />
focused on getting fine detailed images as<br />
early as possible to help clinicians easily<br />
perform confident assessments of fetal<br />
health.<br />
TrueVue : Paired with TrueVue, the<br />
EPIQ Elite system allows clinicians to<br />
manipulate a virtual light source around<br />
the 3D images of the fetus, producing<br />
images that are both incredibly lifelike and<br />
that provide a high level of detail to enable<br />
the identification of any abnormalities<br />
early on in pregnancy.<br />
aBiometry Assist : The Philips aBiometry<br />
Assist application uses anatomical<br />
intelligence to automate time spent<br />
gathering measurements, reducing<br />
examining time.<br />
The combination of a new transducer<br />
and advances in software makes the<br />
assessment and diagnosis of vascular<br />
conditions through easy to interpret<br />
images. Ability to produce tailored<br />
solution for obstetrics and gynecology<br />
helps assess fetal health improving the<br />
early detection of birth defects and<br />
potential complications.<br />
This is a sponsored article. FM editorial holds no responsibility for the information therein.<br />
66 / FUTURE MEDICINE / May 2019
AMRITA CENTRE<br />
FOR ROBOTIC<br />
SURGERY<br />
TRAINING<br />
Started in 2016, the centre has trained<br />
over 250 surgeons from all over India in<br />
robotic assisted surgery<br />
NARRATION: DR SHIVANEE SHAH<br />
Technology in surgery has seen<br />
several leaps of advances in<br />
the past few decades;. from<br />
conventional open surgeries to minimally<br />
invasive procedures such as laparoscopic<br />
techniques, video-assisted thoracoscopic<br />
surgeries and more recently, robotic<br />
assisted minimally invasive surgeries.<br />
These innovations and technological<br />
advances are primarily focused on<br />
improving surgical procedures so that<br />
the final outcome in terms of hospital<br />
stay, procedural time, blood-loss, and<br />
post-operative complications such as<br />
infections and pain, are considerably<br />
reduced.<br />
Robot-assisted minimally invasive<br />
procedures are a fairly recent<br />
development in the field of surgical<br />
science. These types of procedures<br />
mainly involve the use of computer<br />
assistance for performing an array of<br />
surgical manipulations through the<br />
smallest of incisional openings. The<br />
use of robotic assistance has several<br />
known benefits, including improved<br />
procedural dexterity, ergonomics,<br />
safety and ease of surgery, all of which<br />
translate to improved patient outcomes.<br />
Some of the major surgical specialties<br />
that have embraced robotic assisted<br />
procedures include gynecology, urology,<br />
gastroenterology, and thoracic surgery.<br />
In terms of the actual procedure,<br />
one of the primary advantages of using<br />
robotic assistance is the magnification<br />
of the surgical field that is available<br />
to the surgeon. To put this in context,<br />
surgical procedures performed under<br />
conventional techniques do not allow<br />
any magnification of the surgical site,<br />
while moderate magnification (2.5 times)<br />
is obtained in laparoscopic techniques.<br />
However, robotic assisted methods allow<br />
up to 10 times magnification, which<br />
by far is a significant improvement<br />
compared to its predecessors, and also<br />
makes a huge difference in how the<br />
68 / FUTURE MEDICINE / May 2019
I was trained at McGill<br />
University, Montreal,<br />
Canada, and now<br />
it’s my turn to train<br />
others.<br />
Dr Anupama R<br />
Robotic Surgeon and<br />
Professor in Gynecologic<br />
Oncology, Amrita Institute<br />
of Medical Sciences, Kochi<br />
surgeon makes operative decisions<br />
during the procedure. The second and<br />
perhaps the most important advantage<br />
that robotic assistance provides is the<br />
way surgical instruments are negotiated<br />
within the surgical site. In open<br />
surgeries, the surgeon directly holds<br />
the instruments, while in laparoscopic<br />
techniques, the surgeon uses the<br />
instruments by viewing them through a<br />
screen that is connected to a peripheral<br />
camera. However, in robotic assisted<br />
procedures, the surgeon maneuvers the<br />
instruments via a console. This consolebased<br />
control of surgical instruments, in<br />
addition to the enhanced magnification<br />
of the surgical site, provides the surgeon<br />
with an increased ability to be dexterous<br />
and perform difficult surgical maneuvers<br />
with ease.<br />
Simple to learn<br />
Considering the advantages offered<br />
by the newer, minimally invasive<br />
May 2019 / FUTURE MEDICINE / 69
techniques, it would appear that their<br />
acceptance would be widespread.<br />
However, adapting to viewing through<br />
a camera in laparoscopic techniques<br />
and maneuvering instruments through<br />
an indirect console in robotic assisted<br />
techniques is something that needs<br />
skill-based adaptation for the surgeons.<br />
Even leading surgeons <strong>may</strong> be unwilling<br />
to perform procedures involving<br />
newer techniques without training.<br />
A well-known example is that of Dr.<br />
Robert Cerfolio, previously at University<br />
of Alabama and presently Chief of<br />
Clinical Thoracic Surgery, NYU Langone<br />
Center, New York, who is a leader in<br />
the field of thoracic surgery and has<br />
performed thousands of thoracic<br />
surgical procedures. According to Dr<br />
Balasubramoniam K. R., Cardiothoracic<br />
Surgeon at Amrita Institute of Medical<br />
Sciences, Kochi, “Even though Dr<br />
Cerfolio had performed countless<br />
open surgeries, he was initially<br />
unable to adapt to the video-assisted<br />
thoracoscopic surgeries. Nonetheless,<br />
Dr. Cerfolio went on to do thousands<br />
of robotic assisted surgeries and is<br />
currently one of the leading surgeons<br />
utilizing robotic assisted techniques<br />
in his field.” Dr Balasubramoniam<br />
underwent robotic surgery training<br />
at the University of Alabama and has<br />
since then performed over 150 robotic<br />
assisted surgeries in the past few<br />
years at Amrita Hospital. He recognizes<br />
the true benefits of this technology<br />
and highly recommends the need<br />
for training and procuring the skills<br />
required for performing robotic assisted<br />
surgeries.<br />
Such advances in technology<br />
undoubtedly come with unique<br />
challenges in terms of bringing<br />
surgeons up to speed and can require<br />
significant training to develop the<br />
special skill sets required to use<br />
the new technology. While all new<br />
techniques require training, some<br />
techniques are easier to master.<br />
Robotic-assisted techniques are simple<br />
to learn, and due to the significant<br />
improvements and benefits of using<br />
robotic assistance, surgeons worldwide<br />
are embracing this technology.<br />
However, formal training and<br />
competency assessment are required.<br />
Many of our renowned, experienced<br />
Indian surgeons have had to go<br />
abroad to complete training for robotic<br />
assisted surgeries, spending money<br />
and valuable time out of the country. A<br />
training centre in India was a growing<br />
need, and this was accomplished in<br />
2016.<br />
Dr A K K Unni, Professor and Head,<br />
Central Animal Facility, Amrita Institute<br />
of Medical Sciences, Kochi, explained to<br />
me how it all came about over a cup<br />
of tea and cookies. “Intuitive Surgical<br />
Inc. reached out to our Medical Director<br />
to set up a training center at Amrita<br />
hospital. Why Amrita you <strong>may</strong> ask? One<br />
of the major requirements of a training<br />
DUE TO THE BENEFITS OF<br />
USING ROBOTIC ASSISTANCE,<br />
SURGEONS WORLDWIDE<br />
ARE EMBRACING THIS<br />
TECHNOLOGY<br />
centre is an animal facility where<br />
training can be undertaken in large<br />
animals. Amrita hospital already had<br />
a large animal facility and was able to<br />
provide the necessary support required.<br />
Being a leading center for postgraduate<br />
education and research, we also felt<br />
that there was a dire necessity for<br />
training potential surgeons in robotic<br />
surgical techniques.’<br />
The first and until very recently, the<br />
only robotic surgery training center<br />
in India, Amrita Centre for Robotic<br />
Surgery Training and Animal Cath Lab<br />
is a productive collaboration between<br />
Intuitive Surgical Inc., and Amrita Institute<br />
of Medical Sciences. This is a symbiotic<br />
relationship where Intuitive Surgical Inc,<br />
which is the only company that makes<br />
the da Vinci system for robotic assisted<br />
surgeries, provides trainers for the<br />
robotic assistance equipment and Amrita<br />
Hospital provides the infrastructure,<br />
manpower, facilities and the animals<br />
required for training.<br />
Eligibility criteria<br />
Dr Unni has laid out some basic<br />
requirements for the training programme<br />
to ensure that the programme is not<br />
misused. He has put in place a minimum<br />
academic qualification of MS or MCh for<br />
a surgeon to be eligible for this training<br />
programme. The surgeon’s affiliation<br />
hospital must have their own robotic unit<br />
or the surgeon should have prior robotic<br />
surgery exposure. Further, the surgeon’s<br />
institute must give an assurance that<br />
the surgeon would be allowed to pursue<br />
and utilize his training skills after the<br />
training is completed. Once the surgeon<br />
is found to be eligible to undergo the<br />
training programme, he/she must first go<br />
through an online course before visiting<br />
Amrita for a 1-day training session. The<br />
session starts with dry training on the<br />
state of the art da Vinci surgical system<br />
to familiarize the trainee with the system<br />
and the instruments, followed by wet<br />
70 / FUTURE MEDICINE / May 2019
training where surgeons get handson<br />
training on anesthetized pigs. They<br />
undergo training that covers a series of<br />
exercises, including dissection, ligation of<br />
vessels, suturing and energy applications.<br />
At the end of the day, the trainees are<br />
evaluated and then provided a certificate<br />
for this basic training. So far, about 250<br />
surgeons from all over India have been<br />
trained and qualified from the Amrita<br />
Center for Robotic Surgery Training.<br />
The surgeons are also requested to<br />
provide feedback on the training facility<br />
and the programme, and the feedback<br />
has been extremely positive so far.<br />
The training does not end there. All<br />
surgeons who undergo basic training are<br />
recommended to observe real surgeries<br />
before performing their first robotic<br />
procedure on a patient. Then, to finally<br />
complete the training, they are required<br />
to perform 3-4 cases in the presence of<br />
a proctor. There is however no sign off or<br />
certificate provided at this stage.<br />
Cadaver training<br />
As of 2017, In addition to the basic<br />
training in collaboration with Intuitive<br />
Surgical, Amrita also offers an advanced<br />
training programme for specialist<br />
surgeons that involves training on<br />
cadavers. Trained and experienced<br />
robotic surgeons with expertise in<br />
various specialties provide training<br />
for this advanced programme. I was<br />
fortunate to catch Dr Anupama R,<br />
Robotic Surgeon and Professor in<br />
Gynecologic Oncology at Amrita Institute<br />
of Medical Sciences, Kochi, between<br />
surgeries. She was instrumental in setting<br />
up the Minimally Invasive and Robotic<br />
Surgery Programme at Department of<br />
Gynecologic Oncology in February 2015<br />
and since then has performed over 500<br />
robotic surgeries. She has also been<br />
instrumental in the training programme<br />
at Amrita and proctors for other trainees<br />
across the country as well. When<br />
asked how she manages training and<br />
proctoring with her busy schedule, Dr<br />
Anupama says, ‘‘I was trained at McGill<br />
University, Montreal, Canada, and now it’s<br />
my turn to train others.’’<br />
While Amrita Centre for Robotic<br />
Surgery Training remains the only animal<br />
training facility in India, very recently, a<br />
basic training center has been started in<br />
Ramaiah Medical College, Bengaluru. This<br />
new facility is currently equipped only<br />
for training on cadaver models. Animal<br />
training, however, will remain critical for<br />
getting real, hands-on experience as a<br />
first step towards patient surgeries.<br />
Based on trainee conversions and<br />
surgical outcomes in patients, Amrita<br />
Centre for Robotic Surgery Training<br />
has been ranked as the best training<br />
center outside USA by Intuitive Surgicals,<br />
which is a great achievement for all the<br />
management and staff involved. Dr Unni<br />
has set still higher goals and is in talks<br />
with IRCAD, a renowned training center<br />
in France, in an effort to make Amrita<br />
Centre for Robotic Surgery Training one<br />
of the best International training centers<br />
in the world.<br />
This is part of a series that features India’s<br />
First & Most Unique institutions, facilities,<br />
technologies, products etc in the medical and<br />
healthcare space.<br />
May 2019 / FUTURE MEDICINE / 71
guidelines<br />
URINARY<br />
INCONTINENCE<br />
72 / FUTURE MEDICINE / May 2019
PELVIC ORGAN<br />
PROLAPSE IN WOMEN<br />
Urinary incontinence can be a result of functional<br />
abnormalities in the lower urinary tract or of illnesses.<br />
Stress urinary incontinence is involuntary urine leakage<br />
on effort, exertion, sneezing or coughing. Urgency urinary<br />
incontinence is involuntary urine leakage accompanied or<br />
immediately preceded by urgency (a sudden compelling<br />
desire to urinate that is difficult to delay). Mixed urinary<br />
incontinence is involuntary urine leakage associated with<br />
both urgency and exertion, effort, sneezing or coughing.<br />
Overactive bladder (OAB) is defined as urgency that occurs<br />
with or without urgency urinary incontinence and usually with<br />
frequency and nocturia.<br />
Pelvic organ prolapse is defined as symptomatic descent<br />
of 1 or more of the anterior vaginal wall, the posterior vaginal<br />
wall, the cervix or uterus, or the apex of the vagina (vault<br />
or cuff). Symptoms include a vaginal bulge or sensation<br />
of something coming down, urinary, bowel and sexual<br />
symptoms, and pelvic and back pain. These symptoms affect<br />
women's quality of life.<br />
The prevalence of pelvic organ prolapse is high; in primary<br />
care in the UK, 8.4% of women reported vaginal bulge or<br />
lump, and on examination, prolapse is present in up to 50%<br />
of women. One in 10 women will need at least 1 surgical<br />
procedure, and the rate of re‐operation is as high as 19%.<br />
There is likely to be an increasing need for surgery for urinary<br />
incontinence and pelvic organ prolapse because of the ageing<br />
population, according to National Institute for Health and Care<br />
Excellence (NICE),UK.<br />
1.1 Organisation of specialist<br />
services<br />
Local multidisciplinary teams<br />
1.1.1 Local multidisciplinary teams (MDTs)<br />
for women with primary stress urinary<br />
incontinence, overactive bladder or<br />
primary prolapse should:<br />
• review the proposed treatment for<br />
all women offered invasive procedures<br />
for primary stress urinary incontinence,<br />
overactive bladder or primary prolapse<br />
• review the proposed management<br />
for women with primary stress urinary<br />
incontinence, overactive bladder or<br />
primary prolapse if input from a wider<br />
range of healthcare professionals is<br />
needed<br />
• work within an established clinical<br />
network that has access to a regional<br />
MDT.<br />
1.1.2 Local MDTs for women with primary<br />
stress urinary incontinence, overactive<br />
bladder or primary prolapse should<br />
include:<br />
• 2 consultants with expertise in<br />
managing urinary incontinence in<br />
women and/or pelvic organ prolapse<br />
• a urogynaecology, urology or<br />
continence specialist nurse<br />
• a pelvic floor specialist<br />
physiotherapist<br />
• and <strong>may</strong> also include:<br />
• a member of the care of the elderly<br />
team<br />
• an occupational therapist<br />
• a colorectal surgeon.<br />
1.1.3 Members of the local MDT (listed in<br />
recommendation 1.1.2) should attend all<br />
local MDT meetings.<br />
Regional multidisciplinary teams<br />
1.1.4 Regional MDTs that deal with<br />
complex pelvic floor dysfunction and<br />
mesh-related problems should review<br />
the proposed treatment for women if:<br />
• they are having repeat continence<br />
surgery<br />
• they are having repeat, same-site<br />
prolapse surgery<br />
• their preferred treatment option is<br />
not available in the referring hospital<br />
• they have coexisting bowel problems<br />
that <strong>may</strong> need additional colorectal<br />
intervention<br />
• vaginal mesh for prolapse is a<br />
treatment option for them<br />
• they have mesh complications or<br />
unexplained symptoms after mesh<br />
surgery for urinary incontinence or<br />
prolapse<br />
• they are considering surgery and<br />
<strong>may</strong> wish to have children in the<br />
future.<br />
1.1.5 Regional MDTs that deal with<br />
complex pelvic floor dysfunction and<br />
mesh-related problems should include:<br />
• a subspecialist in urogynaecology<br />
• a urologist with expertise in female<br />
urology<br />
• a urogynaecology, urology or<br />
continence specialist nurse<br />
• a pelvic floor specialist<br />
physiotherapist<br />
• a radiologist with expertise in pelvic<br />
floor imaging<br />
• a colorectal surgeon with expertise<br />
in pelvic floor problems<br />
• a pain specialist with expertise in<br />
managing pelvic pain<br />
• and <strong>may</strong> also include:<br />
• a healthcare professional trained<br />
May 2019 / FUTURE MEDICINE / 73
in bowel biofeedback and trans-anal<br />
irrigation<br />
• a clinical psychologist<br />
• a member of the care of the elderly<br />
team<br />
• an occupational therapist<br />
• a surgeon skilled at operating in the<br />
obturator region<br />
• a plastic surgeon.<br />
1.1.6 Regional MDTs that deal with<br />
complex pelvic floor dysfunction and<br />
mesh-related problems should have<br />
ready access to the following services:<br />
• psychology<br />
• psychosexual counselling<br />
• chronic pain management<br />
• bowel symptom management<br />
• neurology.<br />
1.1.7 Members of the regional MDT (listed<br />
in recommendation 1.1.5) should attend<br />
regional MDT meetings when their<br />
specific expertise is needed.<br />
1.2 Collecting data on surgery and<br />
surgical complications<br />
1.2.1 Ask women having surgery<br />
for stress urinary incontinence or<br />
pelvic organ prolapse, or who have<br />
experienced complications related<br />
to these types of surgery, for their<br />
consent to enter the data listed in<br />
recommendation 1.2.2 in a national<br />
registry. Give each woman a copy of her<br />
data.<br />
1.2.2 Providers must ensure that<br />
the following data are recorded in a<br />
national registry of surgery for urinary<br />
incontinence and pelvic organ prolapse<br />
in women:<br />
• the woman's NHS number<br />
• hospital and consultant identifiers<br />
• date and details of the procedure<br />
• for procedures involving mesh, the<br />
mesh material, manufacturer, product<br />
unique identification code and type of<br />
sutures used<br />
• for procedures involving<br />
colposuspension, the type of sutures<br />
used<br />
• for procedures involving bulking<br />
agent, the bulking material,<br />
manufacturer and product unique<br />
identification code<br />
• date and details of any investigation<br />
for complications<br />
• date and details of any surgical<br />
or non-surgical intervention for<br />
complications.<br />
1.2.3 The national registry of surgery for<br />
urinary incontinence and pelvic organ<br />
prolapse in women must ensure that<br />
follow‐up data are collected on key<br />
short- and long-term (at least 5 years)<br />
outcomes, including:<br />
• validated relevant outcome<br />
measures<br />
• adverse events including pain<br />
• suspected and confirmed meshrelated<br />
complications.<br />
1.2.4 The national registry of surgery<br />
for urinary incontinence and pelvic<br />
organ prolapse in women should report<br />
annually and be quality assured.<br />
1.3 Assessing urinary incontinence<br />
History taking and physical<br />
examination<br />
1.3.1 At the initial clinical assessment,<br />
categorise the woman's urinary<br />
incontinence as stress urinary<br />
incontinence, mixed urinary incontinence<br />
or urgency urinary incontinence/<br />
overactive bladder. Start initial<br />
treatment on this basis. In mixed urinary<br />
incontinence, direct treatment towards<br />
the predominant symptom.<br />
1.3.2 If stress incontinence is the<br />
predominant symptom in mixed urinary<br />
incontinence, discuss with the woman<br />
the benefit of non-surgical management<br />
and medicines for overactive bladder<br />
before offering surgery.<br />
1.3.3 During the clinical assessment seek<br />
to identify relevant predisposing and<br />
precipitating factors and other diagnoses<br />
that <strong>may</strong> require referral for additional<br />
investigation and treatment.<br />
Assessing pelvic floor muscles<br />
1.3.4 Undertake routine digital<br />
assessment to confirm pelvic floor<br />
muscle contraction before the use of<br />
supervised pelvic floor muscle training<br />
for the treatment of urinary incontinence.<br />
Urine testing<br />
1.3.5 Undertake a urine dipstick test<br />
in all women presenting with urinary<br />
incontinence to detect the presence of<br />
blood, glucose, protein, leucocytes and<br />
nitrites in the urine.<br />
1.3.6 If women have symptoms of<br />
urinary tract infection (UTI) and their<br />
urine tests positive for both leucocytes<br />
and nitrites, send a midstream urine<br />
specimen for culture and analysis<br />
of antibiotic sensitivities. Prescribe<br />
an appropriate course of antibiotic<br />
treatment pending culture results.<br />
See the NICE guideline on urinary<br />
tract infection (lower): antimicrobial<br />
prescribing for more information.<br />
1.3.7 If women have symptoms of UTI<br />
and their urine tests negative for either<br />
leucocytes or nitrites, send a midstream<br />
urine specimen for culture and analysis<br />
of antibiotic sensitivities. Consider the<br />
prescription of antibiotics pending<br />
culture results.<br />
1.3.8 If women do not have symptoms<br />
of UTI, but their urine tests positive for<br />
both leucocytes and nitrites, do not<br />
offer antibiotics without the results of<br />
midstream urine culture.<br />
1.3.9 If a woman does not have<br />
symptoms of UTI and her urine tests<br />
negative for either leucocytes or nitrites,<br />
do not send a urine sample for culture<br />
because she is unlikely to have UTI.<br />
Assessing residual urine<br />
1.3.10 Measure post-void residual volume<br />
by bladder scan or catheterisation in<br />
women with symptoms suggestive of<br />
voiding dysfunction or recurrent UTI.<br />
1.3.11 Use a bladder scan in preference<br />
to catheterisation on the grounds of<br />
acceptability and lower incidence of<br />
adverse events.<br />
Symptom scoring and quality-of-life<br />
assessment<br />
1.3.12 Use a validated urinary<br />
incontinence-specific symptom and<br />
quality-of-life questionnaire when<br />
therapies are being evaluated.<br />
Bladder diaries<br />
1.3.13 Use bladder diaries in the initial<br />
assessment of women with urinary<br />
incontinence or overactive bladder.<br />
Encourage women to complete a<br />
74 / FUTURE MEDICINE / May 2019
minimum of 3 days of the diary covering<br />
variations in their usual activities, such as<br />
both working and leisure days.<br />
Pad testing<br />
1.3.14 Do not use pad tests in the routine<br />
assessment of women with urinary<br />
incontinence.<br />
Urodynamic testing<br />
1.3.15 Do not perform multichannel<br />
filling and voiding cystometry before<br />
primary surgery if stress urinary<br />
incontinence or stress-predominant<br />
mixed urinary incontinence is diagnosed<br />
based on a detailed clinical history and<br />
demonstrated stress urinary incontinence<br />
at examination.<br />
1.3.16 After undertaking a detailed<br />
clinical history and examination,<br />
perform multichannel filling and voiding<br />
cystometry before surgery for stress<br />
urinary incontinence in women who have<br />
any of the following:<br />
• urge-predominant mixed urinary<br />
incontinence or urinary incontinence in<br />
which the type is unclear<br />
• symptoms suggestive of voiding<br />
dysfunction<br />
• anterior or apical prolapse<br />
• a history of previous surgery for<br />
stress urinary incontinence.<br />
Other tests of urethral competence<br />
1.3.17 Do not use the Q‐tip, Bonney,<br />
Marshall and Fluid-Bridge tests in the<br />
assessment of women with urinary<br />
incontinence.<br />
Cystoscopy<br />
1.3.18 Do not use cystoscopy in the<br />
initial assessment of women with urinary<br />
incontinence alone.<br />
Imaging<br />
1.3.19 Do not use imaging (MRI, CT,<br />
X‐ray) for the routine assessment of<br />
women with urinary incontinence. Do<br />
not use ultrasound other than for the<br />
assessment of residual urine volume.<br />
Indications for referral to a specialist<br />
service<br />
1.3.20 In women with urinary<br />
incontinence, indications for<br />
consideration for referral to a specialist<br />
service include:<br />
• persisting bladder or urethral pain<br />
• palpable bladder on bimanual or<br />
abdominal examination after voiding<br />
• clinically benign pelvic masses<br />
• associated faecal incontinence<br />
• suspected neurological disease<br />
• symptoms of voiding difficulty<br />
• suspected urogenital fistulae<br />
• previous continence surgery<br />
• previous pelvic cancer surgery<br />
• previous pelvic radiation therapy.<br />
1.3.21 Follow the recommendations on<br />
referral for urinary tract cancer in the<br />
NICE guideline on suspected cancer, for<br />
women with haematuria or recurrent or<br />
persistent unexplained UTI.<br />
1.4 Non-surgical management of<br />
ADVISE WOMEN WITH<br />
URINARY INCONTINENCE OR<br />
OVERACTIVE BLADDER WHO<br />
HAVE A BMI GREATER THAN<br />
30 TO LOSE WEIGHT<br />
urinary incontinence<br />
Lifestyle interventions<br />
1.4.1 Recommend a trial of caffeine<br />
reduction to women with overactive<br />
bladder.<br />
1.4.2 Consider advising women with<br />
urinary incontinence or overactive<br />
bladder and a high or low fluid intake to<br />
modify their fluid intake.<br />
1.4.3 Advise women with urinary<br />
incontinence or overactive bladder who<br />
have a BMI greater than 30 to lose<br />
weight.<br />
Physical therapies<br />
Pelvic floor muscle training<br />
1.4.4 Offer a trial of supervised pelvic<br />
floor muscle training of at least 3<br />
months' duration as first-line treatment<br />
to women with stress or mixed urinary<br />
incontinence.<br />
1.4.5 Pelvic floor muscle training<br />
programmes should comprise at least 8<br />
contractions performed 3 times per day.<br />
1.4.6 Do not use perineometry or pelvic<br />
floor electromyography as biofeedback<br />
as a routine part of pelvic floor muscle<br />
training.<br />
1.4.7 Continue an exercise programme if<br />
pelvic floor muscle training is beneficial.<br />
Electrical stimulation<br />
1.4.8 Do not routinely use electrical<br />
stimulation in the treatment of women<br />
with overactive bladder.<br />
1.4.9 Do not routinely use electrical<br />
stimulation in combination with pelvic<br />
floor muscle training.<br />
1.4.10 Electrical stimulation and/or<br />
biofeedback should be considered for<br />
women who cannot actively contract<br />
pelvic floor muscles to aid motivation<br />
and adherence to therapy.<br />
Behavioural therapies<br />
1.4.11 Offer bladder training lasting<br />
for a minimum of 6 weeks as first-line<br />
treatment to women with urgency or<br />
mixed urinary incontinence.<br />
1.4.12 If women do not achieve<br />
satisfactory benefit from bladder<br />
training programmes, the combination<br />
of an overactive bladder medicine with<br />
bladder training should be considered if<br />
frequency is a troublesome symptom.<br />
Neurostimulation<br />
1.4.13 Do not offer transcutaneous sacral<br />
nerve stimulation (surface electrodes<br />
placed above the sacrum, often known<br />
as transcutaneous electrical nerve<br />
stimulation [TENS]) to treat overactive<br />
bladder in women.<br />
1.4.14 Do not offer transcutaneous<br />
posterior tibial nerve stimulation for<br />
overactive bladder.<br />
1.4.15 Do not offer percutaneous<br />
posterior tibial nerve stimulation (needles<br />
inserted close to the posterior tibial<br />
nerve) for overactive bladder unless:<br />
• there has been a local MDT review<br />
and<br />
• non-surgical management including<br />
overactive bladder medicine treatment<br />
has not worked adequately and<br />
May 2019 / FUTURE MEDICINE / 75
slug<br />
• the woman does not want<br />
botulinum toxin type A or<br />
percutaneous sacral nerve stimulation.<br />
Absorbent containment products,<br />
urinals and toileting aids<br />
1.4.16 Do not offer absorbent<br />
containment products, hand-held<br />
urinals or toileting aids to treat urinary<br />
incontinence. Offer them only:<br />
• as a coping strategy pending<br />
definitive treatment<br />
• as an adjunct to ongoing therapy<br />
• for long-term management of<br />
urinary incontinence only after<br />
treatment options have been explored.<br />
1.4.17 Offer a review at least once a year<br />
to women who are using absorbent<br />
containment products for long-term<br />
management of urinary incontinence.<br />
The review should cover:<br />
• routine assessment of continence<br />
• assessment of skin integrity<br />
• changes to symptoms, comorbidities,<br />
lifestyle, mobility, medication, BMI, and<br />
social and environmental factors<br />
• the suitability of alternative<br />
treatment options<br />
• the efficacy of the absorbent<br />
containment product the woman is<br />
currently using and the quantities<br />
used.<br />
1.4.18 Reviews for women who are<br />
using absorbent containment products<br />
for long-term management of urinary<br />
incontinence should be carried out by<br />
either:<br />
• a registered healthcare professional<br />
who is trained in assessing continence<br />
and making referrals to specialist<br />
services or<br />
• a non-registered healthcare worker,<br />
under the supervision of a registered<br />
healthcare professional who is trained<br />
in assessing continence and making<br />
referrals to specialist services.<br />
Catheters<br />
1.4.19 Bladder catheterisation<br />
(intermittent or indwelling urethral or<br />
suprapubic) should be considered for<br />
women in whom persistent urinary<br />
retention is causing incontinence,<br />
symptomatic infections or renal<br />
dysfunction, and in whom this cannot<br />
otherwise be corrected. Healthcare<br />
professionals should be aware, and<br />
explain to women, that the use<br />
of indwelling catheters in urgency<br />
urinary incontinence <strong>may</strong> not result in<br />
continence.<br />
1.4.20 Offer intermittent urethral<br />
catheterisation to women with urinary<br />
retention who can be taught to selfcatheterise<br />
or who have a carer who can<br />
perform the technique.<br />
1.4.21 Give careful consideration to the<br />
impact of long-term indwelling urethral<br />
catheterisation. Discuss the practicalities,<br />
benefits and risks with the woman or,<br />
if appropriate, her carer. Indications for<br />
the use of long-term indwelling urethral<br />
catheters for women with urinary<br />
incontinence include:<br />
• chronic urinary retention in<br />
OFFER INTERMITTENT<br />
URETHRAL CATHETERISATION<br />
TO WOMEN WITH URINARY<br />
RETENTION WHO CAN<br />
BE TAUGHT TO SELF-<br />
CATHETERISE OR WHO HAVE<br />
A CARER WHO CAN PERFORM<br />
THE TECHNIQUE<br />
women who are unable to manage<br />
intermittent self-catheterisation<br />
• skin wounds, pressure ulcers or<br />
irritations that are being contaminated<br />
by urine<br />
• distress or disruption caused by bed<br />
and clothing changes<br />
• where a woman expresses<br />
a preference for this form of<br />
management.<br />
1.4.22 Indwelling suprapubic catheters<br />
should be considered as an alternative<br />
to long-term urethral catheters. Be<br />
aware, and explain to women, that they<br />
<strong>may</strong> be associated with lower rates<br />
of symptomatic UTI, 'bypassing', and<br />
urethral complications than indwelling<br />
76 / FUTURE MEDICINE / May 2019
urethral catheters.<br />
Products to prevent leakage<br />
1.4.23 Do not use intravaginal and<br />
intraurethral devices for the routine<br />
management of urinary incontinence<br />
in women. Do not advise women to<br />
consider such devices other than for<br />
occasional use when necessary to<br />
prevent leakage, for example during<br />
physical exercise.<br />
Complementary therapies<br />
1.4.24 Do not recommend<br />
complementary therapies for the<br />
treatment of urinary incontinence or<br />
overactive bladder.<br />
Medicines for overactive bladder<br />
1.4.25 Before starting treatment with a<br />
medicine for overactive bladder, explain<br />
to the woman:<br />
• the likelihood of the medicine being<br />
successful<br />
• the common adverse effects<br />
associated with the medicine<br />
• that some adverse effects of<br />
anticholinergic medicines, such as dry<br />
mouth and constipation, <strong>may</strong> indicate<br />
that the medicine is starting to have<br />
an effect<br />
• that she <strong>may</strong> not see substantial<br />
benefits until she has been taking the<br />
medicine for at least 4 weeks and<br />
that her symptoms <strong>may</strong> continue to<br />
improve over time<br />
• that the long-term effects of<br />
anticholinergic medicines for<br />
overactive bladder on cognitive<br />
function are uncertain.<br />
1.4.26 When offering anticholinergic<br />
medicines to treat overactive bladder,<br />
take account of the woman's:<br />
• coexisting conditions (such as<br />
poor bladder emptying, cognitive<br />
impairment or dementia)<br />
• current use of other medicines that<br />
affect total anticholinergic load<br />
• risk of adverse effects, including<br />
cognitive impairment.<br />
1.4.27 For women who have a<br />
diagnosis of dementia and for whom<br />
anticholinergic medicines are an<br />
option, follow the recommendations<br />
May 2019 / FUTURE MEDICINE / 77
on medicines that <strong>may</strong> cause cognitive<br />
impairment in the NICE guideline on<br />
dementia.<br />
Choosing medicine<br />
1.4.28 Do not offer women flavoxate,<br />
propantheline or imipramine to treat<br />
urinary incontinence or overactive<br />
bladder.<br />
1.4.29 Do not offer oxybutynin<br />
(immediate release) to older women<br />
who <strong>may</strong> be at higher risk of a sudden<br />
deterioration in their physical or mental<br />
health.<br />
1.4.30 Offer the anticholinergic medicine<br />
with the lowest acquisition cost to treat<br />
overactive bladder or mixed urinary<br />
incontinence in women.<br />
1.4.31 If the first medicine for overactive<br />
bladder or mixed urinary incontinence<br />
is not effective or well-tolerated, offer<br />
another medicine with a low acquisition<br />
cost.<br />
1.4.32 Offer a transdermal overactive<br />
bladder treatment to women unable to<br />
tolerate oral medicines.<br />
1.4.33 For guidance on mirabegron, see<br />
the NICE technology appraisal guidance<br />
on mirabegron for treating symptoms of<br />
overactive bladder.<br />
1.4.34 The use of desmopressin <strong>may</strong> be<br />
considered specifically to reduce nocturia<br />
in women with urinary incontinence<br />
or overactive bladder who find it a<br />
troublesome symptom. Use particular<br />
caution in women with cystic fibrosis<br />
and avoid in those over 65 years with<br />
cardiovascular disease or hypertension.<br />
1.4.35 Do not use duloxetine as a<br />
first-line treatment for women with<br />
predominant stress urinary incontinence.<br />
Do not routinely offer duloxetine as a<br />
second-line treatment for women with<br />
stress urinary incontinence, although it<br />
<strong>may</strong> be offered as second-line therapy<br />
if women prefer pharmacological to<br />
surgical treatment or are not suitable<br />
for surgical treatment. If duloxetine is<br />
prescribed, counsel women about its<br />
adverse effects.<br />
1.4.36 Do not offer systemic hormone<br />
replacement therapy to treat urinary<br />
incontinence.<br />
1.4.37 Offer intravaginal oestrogens to<br />
treat overactive bladder symptoms in<br />
postmenopausal women with vaginal<br />
atrophy.<br />
Reviewing medicine<br />
1.4.38 Offer a face-to-face or telephone<br />
review 4 weeks after starting a new<br />
medicine for overactive bladder. Ask<br />
the woman if she is satisfied with the<br />
treatment and:<br />
• if improvement is optimal, continue<br />
treatment<br />
• if there is no or suboptimal<br />
improvement, or intolerable adverse<br />
effects, change the dose or try an<br />
alternative medicine for overactive<br />
THE USE OF DESMOPRESSIN<br />
MAY BE CONSIDERED<br />
SPECIFICALLY TO REDUCE<br />
NOCTURIA IN WOMEN WITH<br />
URINARY INCONTINENCE WHO<br />
FIND IT A TROUBLESOME<br />
SYMPTOM<br />
bladder (see recommendations 1.4.31<br />
and 1.4.32), and review again 4 weeks<br />
later.<br />
1.4.39 Offer a review before 4 weeks<br />
if the adverse events of a medicine for<br />
overactive bladder are intolerable.<br />
1.4.40 Refer women who have tried<br />
taking medicine for overactive bladder,<br />
but for whom it has not been successful<br />
or tolerated, to secondary care to<br />
consider further treatment.<br />
1.4.41 Offer a further face-to-face<br />
or telephone review if a medicine<br />
for overactive bladder or urinary<br />
incontinence stops working after an<br />
initial successful 4‐week review.<br />
1.4.42 Offer a review in primary care<br />
to women who remain on long-term<br />
medicine for overactive bladder or<br />
urinary incontinence every 12 months, or<br />
every 6 months if they are aged over 75.<br />
Invasive procedures for overactive<br />
bladder<br />
1.4.43 For women with overactive<br />
bladder that has not responded to<br />
non-surgical management or treatment<br />
with medicine and who wish to discuss<br />
further treatment options:<br />
78 / FUTURE MEDICINE / May 2019
• offer urodynamic investigation<br />
to determine whether detrusor<br />
overactivity is causing her overactive<br />
bladder symptoms and<br />
• if detrusor overactivity is causing<br />
her overactive bladder symptoms,<br />
offer an invasive procedure in line with<br />
recommendations 1.4.44 to 1.4.56 or<br />
• if there is no detrusor overactivity,<br />
seek advice on further management<br />
from the local MDT in line with<br />
recommendation 1.4.45.<br />
Botulinum toxin type A injection<br />
1.4.44 After a local MDT review, offer<br />
bladder wall injection with botulinum<br />
toxin type A to women with overactive<br />
bladder caused by detrusor overactivity<br />
that has not responded to non-surgical<br />
management, including pharmacological<br />
treatments.<br />
1.4.45 Consider treatment with<br />
botulinum toxin type A after a local MDT<br />
review for women with symptoms of<br />
overactive bladder in whom urodynamic<br />
investigation has not demonstrated<br />
detrusor overactivity, if the symptoms<br />
have not responded to non-surgical<br />
management and the woman does not<br />
wish to have other invasive treatments.<br />
1.4.46 After a local MDT review, discuss<br />
the benefits and risks of treatment with<br />
botulinum toxin type A with the woman<br />
and explain:<br />
• the likelihood of complete or partial<br />
symptom relief<br />
• the process of clean intermittent<br />
catheterisation, the risks, and how<br />
long it might need to be continued<br />
• the risk of adverse effects, including<br />
an increased risk of urinary tract<br />
infection<br />
• that there is not much evidence<br />
about how long the injections work for,<br />
how well they work in the long term<br />
and their long-term risks.<br />
1.4.47 Start treatment with botulinum<br />
toxin type A only if the woman is willing,<br />
in the event of developing significant<br />
voiding dysfunction:<br />
• to perform clean intermittent<br />
catheterisation on a regular basis for<br />
as long as needed or<br />
• to accept a temporary indwelling<br />
catheter if she is unable to perform<br />
clean intermittent catheterisation.<br />
1.4.48 Use 100 units as the initial dose<br />
of botulinum toxin type A to treat<br />
CONSIDER TREATMENT<br />
WITH BOTULINUM TOXIN<br />
TYPE A AFTER A LOCAL<br />
MDT REVIEW FOR WOMEN<br />
WITH SYMPTOMS OF<br />
OVERACTIVE BLADDER<br />
overactive bladder in women.<br />
1.4.49 Offer a face-to-face or telephone<br />
review within 12 weeks of the first<br />
treatment with botulinum toxin type A<br />
to assess the response to treatment and<br />
adverse effects, and:<br />
• if there is good symptom relief,<br />
tell the woman how to self-refer for<br />
prompt specialist review if symptoms<br />
return, and offer repeat treatment as<br />
necessary<br />
• if there is inadequate symptom relief,<br />
consider increasing subsequent doses<br />
of botulinum toxin type A to 200 units<br />
and review within 12 weeks<br />
• if there was no effect, discuss with<br />
the local MDT.<br />
1.4.50 If symptom relief has been<br />
adequate after injection of 100 units of<br />
botulinum toxin type A but has lasted for<br />
less than 6 months, consider increasing<br />
subsequent doses of botulinum toxin<br />
type A to 200 units and review within 12<br />
weeks.<br />
1.4.51 Do not offer botulinum toxin type<br />
B to women with overactive bladder.<br />
Percutaneous sacral nerve stimulation<br />
1.4.52 Offer percutaneous sacral<br />
nerve stimulation to women after<br />
local or regional MDT review if their<br />
overactive bladder has not responded<br />
to non-surgical management including<br />
medicines and:<br />
• their symptoms have not responded<br />
to botulinum toxin type A or<br />
• they are not prepared to accept<br />
the risks of needing catheterisation<br />
associated with botulinum toxin type<br />
A.<br />
1.4.53 Discuss the long-term implications<br />
of percutaneous sacral nerve stimulation<br />
with women including:<br />
• the need for test stimulation and<br />
probability of the test's success<br />
• the risk of failure<br />
• the long-term commitment<br />
• the need for surgical revision<br />
• the adverse effects.<br />
1.4.54 Tell women how to self-refer for<br />
prompt specialist review if symptoms<br />
return following a percutaneous sacral<br />
nerve stimulation procedure.<br />
Augmentation cystoplasty<br />
1.4.55 Restrict augmentation cystoplasty<br />
for the management of idiopathic<br />
detrusor overactivity to women whose<br />
condition has not responded to nonsurgical<br />
management and who are<br />
willing and able to self-catheterise.<br />
Preoperative counselling for the<br />
woman or her carer should include<br />
common and serious complications:<br />
bowel disturbance, metabolic acidosis,<br />
May 2019 / FUTURE MEDICINE / 79
mucus production and/or retention in<br />
the bladder, UTI and urinary retention.<br />
Discuss the small risk of malignancy<br />
occurring in the augmented bladder.<br />
Provide life-long follow‐up.<br />
Urinary diversion<br />
1.4.56 Urinary diversion should be<br />
considered for a woman with overactive<br />
bladder only when non-surgical<br />
management has failed, and if botulinum<br />
toxin type A, percutaneous sacral<br />
nerve stimulation and augmentation<br />
cystoplasty are not appropriate or are<br />
unacceptable to her. Provide life-long<br />
follow‐up.<br />
1.5 Surgical management of stress<br />
urinary incontinence<br />
There is public concern about the use<br />
of mesh procedures. For all of the<br />
procedures recommended in this section,<br />
including mesh procedures, there is<br />
evidence of benefit but limited evidence<br />
on the long-term adverse effects. In<br />
particular, the true prevalence of longterm<br />
complications is unknown.<br />
1.5.1 If a woman is thinking about a<br />
surgical procedure for stress urinary<br />
incontinence, use the NICE patient<br />
decision aid on surgery for stress urinary<br />
incontinence to promote informed<br />
preference and shared decision making.<br />
Discussion with the woman should<br />
include:<br />
• the benefits and risks of all surgical<br />
treatment options for stress urinary<br />
incontinence that NICE recommends,<br />
whether or not they are available<br />
locally<br />
• the uncertainties about the longterm<br />
adverse effects for all procedures,<br />
particularly those involving the<br />
implantation of mesh materials<br />
• differences between procedures<br />
in the type of anaesthesia, expected<br />
length of hospital stay, surgical<br />
incisions and expected recovery period<br />
• any social or psychological factors<br />
that <strong>may</strong> affect the woman's decision.<br />
1.5.2 If non-surgical management<br />
for stress urinary incontinence has<br />
failed, and the woman wishes to think<br />
about a surgical procedure, offer<br />
her the choice of:<br />
• colposuspension (open or<br />
laparoscopic) or<br />
• an autologous rectus fascial sling.<br />
Also include the option of a retropubic<br />
mid-urethral mesh sling in this<br />
choice but see recommendations<br />
1.5.7 to 1.5.11 for additional guidance<br />
on the use of mid-urethral mesh<br />
sling procedures for stress urinary<br />
incontinence.<br />
1.5.3 Consider intramural bulking agents<br />
to manage stress urinary incontinence if<br />
alternative surgical procedures are not<br />
suitable for or acceptable to the woman.<br />
Explain to the woman that:<br />
• these are permanent injectable<br />
materials<br />
• repeat injections <strong>may</strong> be needed to<br />
achieve effectiveness<br />
• limited evidence suggests that they<br />
DO NOT OFFER A<br />
TRANSOBTURATOR<br />
APPROACH UNLESS THERE<br />
ARE SPECIFIC CLINICAL<br />
CIRCUMSTANCES<br />
are less effective than the surgical<br />
procedures listed in recommendation<br />
1.5.2 and the effects wear off over<br />
time<br />
• there is limited evidence on longterm<br />
effectiveness and adverse events.<br />
1.5.4 If an intramural bulking agent<br />
is injected, give the woman written<br />
information about the bulking agent,<br />
including its name, manufacturer, date<br />
of injection, and the injecting surgeon's<br />
name and contact details.<br />
1.5.5 If the woman's chosen procedure<br />
for stress urinary incontinence is not<br />
available from the consulting surgeon,<br />
refer her to an alternative surgeon.<br />
1.5.6 Providers must ensure that data<br />
on surgical procedures for stress urinary<br />
incontinence are recorded in a national<br />
registry, as outlined in the section on<br />
collecting data on surgery and surgical<br />
complications in this guideline.<br />
Mid-urethral mesh sling procedures<br />
1.5.7 When offering a retropubic midurethral<br />
mesh sling, advise the woman<br />
that it is a permanent implant and<br />
complete removal might not be possible.<br />
1.5.8 If a retropubic mid-urethral mesh<br />
sling is inserted, give the woman written<br />
information about the implant, including<br />
its name, manufacturer, date of insertion,<br />
and the implanting surgeon's name and<br />
contact details.<br />
1.5.9 When planning a retropubic midurethral<br />
mesh sling procedure, surgeons<br />
should:<br />
• use a device manufactured from<br />
type 1 macroporous polypropylene<br />
mesh<br />
• consider using a retropubic midurethral<br />
mesh sling coloured for high<br />
visibility, for ease of insertion and<br />
revision.<br />
1.5.10 Do not offer a transobturator<br />
approach unless there are specific<br />
clinical circumstances (for example,<br />
previous pelvic procedures) in which the<br />
retropubic approach should be avoided.<br />
1.5.11 Do not use the 'top‐down'<br />
retropubic mid-urethral mesh sling<br />
approach or single-incision sub-urethral<br />
short mesh sling insertion except as part<br />
of a clinical trial.<br />
Artificial urinary sphincters<br />
1.5.12 Do not offer women an artificial<br />
urinary sphincter to manage stress<br />
urinary incontinence unless previous<br />
surgery has failed.<br />
1.5.13 For women who have had an<br />
artificial urinary sphincter inserted:<br />
• offer postoperative follow‐up and<br />
• ensure access to review if needed.<br />
Procedures that should not be offered<br />
1.5.14 Do not offer women the following<br />
procedures to treat stress urinary<br />
incontinence:<br />
• anterior colporrhaphy<br />
• needle suspension<br />
• paravaginal defect repair<br />
• porcine dermis sling<br />
80 / FUTURE MEDICINE / May 2019
• the Marshall–Marchetti–Krantz<br />
procedure.<br />
Follow-up after surgery<br />
1.5.15 Offer a follow‐up appointment<br />
within 6 months to all women who have<br />
had a surgical procedure to treat stress<br />
urinary incontinence.<br />
1.5.16 For women who have had<br />
retropubic mid-urethral mesh sling<br />
surgery, the follow‐up appointment<br />
should include a vaginal examination to<br />
check for exposure or extrusion of the<br />
mesh sling.<br />
1.5.17 Providers should ensure that<br />
women who have had surgery for stress<br />
urinary incontinence have access to<br />
further referral if they have recurrent<br />
symptoms or suspected complications.<br />
See also assessing complications<br />
associated with mesh surgery in this<br />
guideline.<br />
1.5.18 For women whose primary surgical<br />
procedure for stress urinary incontinence<br />
has failed (including women whose<br />
symptoms have returned):<br />
• seek advice on assessment and<br />
management from a regional MDT<br />
that deals with complex pelvic floor<br />
dysfunction or<br />
• offer the woman advice about<br />
managing urinary symptoms if she<br />
does not wish to have another surgical<br />
procedure, and explain that she can<br />
ask for a referral if she changes her<br />
mind.<br />
1.6 Assessing pelvic organ prolapse<br />
1.6.1 For women presenting in primary<br />
care with symptoms or an incidental<br />
finding of vaginal prolapse:<br />
• take a history to include symptoms<br />
of prolapse, urinary, bowel and sexual<br />
function<br />
• do an examination to rule out a<br />
pelvic mass or other pathology and to<br />
document the presence of prolapse<br />
(see the sections on ovarian cancer<br />
and bladder cancer in the NICE<br />
guideline on suspected cancer)<br />
• discuss the woman's treatment<br />
preferences with her, and refer if<br />
needed.<br />
1.6.2 For women referred to secondary<br />
care for an unrelated condition who have<br />
incidental symptoms or an incidental<br />
finding of vaginal prolapse, consider<br />
referral to a clinician with expertise in<br />
prolapse.<br />
1.6.3 For women who are referred for<br />
specialist evaluation of vaginal prolapse,<br />
perform an examination to:<br />
• assess and record the presence<br />
and degree of prolapse of the<br />
anterior, central and posterior vaginal<br />
compartments of the pelvic floor, using<br />
the POP‐Q (Pelvic Organ Prolapse<br />
Quantification) system<br />
• assess the activity of the pelvic floor<br />
muscles<br />
• assess for vaginal atrophy<br />
• rule out a pelvic mass or other<br />
pathology.<br />
1.6.4 For women with pelvic organ<br />
prolapse, consider using a validated<br />
pelvic floor symptom questionnaire to<br />
aid assessment and decision making.<br />
1.6.5 Do not routinely perform imaging<br />
to document the presence of vaginal<br />
prolapse if a prolapse is detected by<br />
physical examination.<br />
1.6.6 If the woman has symptoms of<br />
prolapse that are not explained by<br />
findings from a physical examination,<br />
consider repeating the examination with<br />
the woman standing or squatting, or at a<br />
different time.<br />
1.6.7 Consider investigating the following<br />
symptoms in women with pelvic organ<br />
prolapse:<br />
• urinary symptoms that are<br />
bothersome and for which surgical<br />
intervention is an option<br />
• symptoms of obstructed defaecation<br />
or faecal incontinence (the NICE<br />
guideline on faecal incontinence<br />
in adults has recommendations<br />
on baseline assessment of faecal<br />
incontinence)<br />
May 2019 / FUTURE MEDICINE / 81
• pain<br />
• symptoms that are not explained by<br />
examination findings.<br />
1.7 Non-surgical management of<br />
pelvic organ prolapse<br />
1.7.1 Discuss management options with<br />
women who have pelvic organ prolapse,<br />
including no treatment, non-surgical<br />
treatment and surgical options, taking<br />
into account:<br />
• the woman's preferences<br />
• site of prolapse<br />
• lifestyle factors<br />
• comorbidities, including cognitive or<br />
physical impairments<br />
• age<br />
• desire for childbearing<br />
• previous abdominal or pelvic floor<br />
surgery<br />
• benefits and risks of individual<br />
procedures.<br />
Lifestyle modification<br />
1.7.2 Consider giving advice on lifestyle<br />
to women with pelvic organ prolapse,<br />
including information on:<br />
• losing weight, if the woman has a<br />
BMI greater than 30 kg/m2<br />
• minimising heavy lifting<br />
• preventing or treating constipation.<br />
Topical oestrogen<br />
1.7.3 Consider vaginal oestrogen for<br />
women with pelvic organ prolapse<br />
and signs of vaginal atrophy. For<br />
recommendations on managing<br />
urogenital atrophy, see managing shortterm<br />
menopausal symptoms in the NICE<br />
guideline on menopause.<br />
1.7.4 Consider an oestrogen-releasing<br />
ring for women with pelvic organ<br />
prolapse and signs of vaginal atrophy<br />
who have cognitive or physical<br />
impairments that might make vaginal<br />
oestrogen pessaries or creams difficult<br />
to use.<br />
Pelvic floor muscle training<br />
1.7.5 Consider a programme of<br />
supervised pelvic floor muscle training<br />
for at least 16 weeks as a first option for<br />
women with symptomatic POP‐Q (Pelvic<br />
Organ Prolapse Quantification) stage 1<br />
or stage 2 pelvic organ prolapse. If the<br />
programme is beneficial, advise women<br />
to continue pelvic floor muscle training<br />
afterwards.<br />
Pessaries<br />
1.7.6 Consider a vaginal pessary for<br />
women with symptomatic pelvic organ<br />
prolapse, alone or in conjunction with<br />
supervised pelvic floor muscle training.<br />
1.7.7 Refer women who have chosen a<br />
pessary to a urogynaecology service if<br />
pessary care is not available locally.<br />
1.7.8 Before starting pessary treatment:<br />
• consider treating vaginal atrophy<br />
with topical oestrogen<br />
• explain that more than 1 pessary<br />
fitting <strong>may</strong> be needed to find a<br />
suitable pessary<br />
• discuss the effect of different types<br />
of pessary on sexual intercourse<br />
• describe complications including<br />
vaginal discharge, bleeding, difficulty<br />
removing pessary and pessary<br />
expulsion<br />
• explain that the pessary should<br />
be removed at least once every 6<br />
months to prevent serious pessary<br />
complications.<br />
1.7.9 Offer women using pessaries<br />
an appointment in a pessary clinic<br />
every 6 months if they are at risk of<br />
complications, for example because<br />
of a physical or cognitive impairment<br />
that might make it difficult for them to<br />
manage their ongoing pessary care.<br />
1.8 Surgical management of pelvic<br />
organ prolapse<br />
There is public concern about the use<br />
of mesh procedures. For all of the<br />
procedures recommended in this section,<br />
including mesh procedures, there is<br />
some evidence of benefit, but limited<br />
evidence on long-term effectiveness and<br />
adverse effects. In particular, the true<br />
prevalence of long-term complications is<br />
unknown.<br />
1.8.1 Offer surgery for pelvic organ<br />
prolapse to women whose symptoms<br />
have not improved with or who have<br />
declined non-surgical treatment.<br />
1.8.2 If a woman is thinking about a<br />
surgical procedure for pelvic organ<br />
prolapse, use a decision aid (use the<br />
NICE patient decision aids on surgery<br />
for uterine prolapse and surgery for<br />
vaginal vault prolapse where they apply)<br />
to promote informed preference and<br />
shared decision making. Discussion with<br />
the woman should include:<br />
• the different treatment options for<br />
pelvic organ prolapse, including no<br />
treatment or continued non-surgical<br />
management<br />
• the benefits and risks of each<br />
surgical procedure, including changes<br />
in urinary, bowel and sexual function<br />
• the risk of recurrent prolapse<br />
• the uncertainties about the longterm<br />
adverse effects for all procedures,<br />
particularly those involving the<br />
implantation of mesh materials<br />
• differences between procedures<br />
in the type of anaesthesia, expected<br />
length of hospital stay, surgical<br />
incisions and expected recovery period<br />
• the role of intraoperative prolapse<br />
assessment in deciding the most<br />
appropriate surgical procedure.<br />
1.8.3 Do not offer surgery to prevent<br />
incontinence in women having<br />
surgery for prolapse who do not have<br />
incontinence.<br />
1.8.4 Explain to women considering<br />
surgery for anterior or apical prolapse<br />
who do not have incontinence that there<br />
is a risk of developing postoperative<br />
urinary incontinence and further<br />
treatment <strong>may</strong> be needed.<br />
1.8.5 If the woman's chosen procedure<br />
for pelvic organ prolapse is not available<br />
from the consulting surgeon, refer her to<br />
an alternative surgeon.<br />
1.8.6 If mesh is to be used in prolapse<br />
surgery:<br />
• explain to the woman about the<br />
type of mesh that will be used and<br />
whether or not it is permanent<br />
• ensure that details of the procedure<br />
and its subsequent short- and longterm<br />
outcomes are recorded in a<br />
national registry (see collecting data<br />
on surgery and surgical complications<br />
in this guideline)<br />
• give the woman written information<br />
about the implant, including its name,<br />
manufacturer, date of insertion, and<br />
82 / FUTURE MEDICINE / May 2019
the implanting surgeon's name and<br />
contact details.<br />
1.8.7 Providers must ensure that data<br />
on surgical procedures for pelvic organ<br />
prolapse are recorded in a national<br />
registry, as outlined in the section on<br />
collecting data on surgery and surgical<br />
complications in this guideline.<br />
Surgery for uterine prolapse<br />
1.8.8 Discuss the options for treatment<br />
(see recommendation 1.8.2), including<br />
non-surgical options, hysterectomy and<br />
surgery that will preserve the uterus,<br />
with women who have uterine prolapse.<br />
1.8.9 For women considering surgery for<br />
uterine prolapse:<br />
• discuss the possible complications<br />
and the lack of long-term evidence on<br />
the effectiveness of the procedures<br />
• use the NICE patient decision aid on<br />
surgery for uterine prolapse to discuss<br />
the benefits and risks of treatment,<br />
including non-surgical options.<br />
1.8.10 For women with uterine prolapse<br />
who have no preference about<br />
preserving their uterus, offer a choice of:<br />
• vaginal hysterectomy, with or<br />
without vaginal sacrospinous fixation<br />
with sutures or<br />
• vaginal sacrospinous hysteropexy<br />
with sutures or<br />
• Manchester repair.<br />
Also include the option of sacrohysteropexy<br />
with mesh (abdominal<br />
or laparoscopic) in this choice but see<br />
recommendation 1.8.6 for specific<br />
guidance on the use of mesh in prolapse<br />
surgery.<br />
1.8.11 For women with uterine prolapse<br />
who wish to preserve their uterus, offer<br />
a choice of:<br />
• vaginal sacrospinous hysteropexy<br />
with sutures or<br />
• Manchester repair, unless the<br />
woman <strong>may</strong> wish to have children in<br />
the future.<br />
Also include the option of sacrohysteropexy<br />
with mesh (abdominal<br />
or laparoscopic) in this choice but see<br />
recommendation 1.8.6 for specific<br />
guidance on the use of mesh in prolapse<br />
surgery.<br />
1.8.12 If a synthetic polypropylene mesh<br />
is inserted, the details of the procedure<br />
and its subsequent short- and longterm<br />
outcomes must be collected in a<br />
national registry (see collecting data on<br />
surgery and surgical complications in this<br />
guideline).<br />
1.8.13 Ensure the proposed treatment<br />
is reviewed by a regional MDT (see<br />
recommendation 1.1.4) if the woman<br />
wishes to have children in the future.<br />
Surgery for vault prolapse<br />
1.8.14 Discuss the options for treatment<br />
(see recommendation 1.8.2), including<br />
non-surgical and surgical options, with<br />
women who have vault prolapse.<br />
1.8.15 For women considering surgery for<br />
vault prolapse:<br />
• discuss the possible complications<br />
A SYNTHETIC<br />
POLYPROPYLENE MESH IS<br />
INSERTED, THE DETAILS OF<br />
THE PROCEDURE AND ITS<br />
SUBSEQUENT SHORT- AND<br />
LONG-TERM OUTCOMES MUST<br />
BE COLLECTED<br />
and the lack of long-term evidence on<br />
the effectiveness of the procedures<br />
• use the NICE patient decision aid<br />
on surgery for vaginal vault prolapse<br />
to discuss the benefits and risks of<br />
treatment, including non-surgical<br />
options.<br />
1.8.16 Offer women with vault prolapse a<br />
choice of:<br />
• vaginal sacrospinous fixation with<br />
sutures or<br />
• sacrocolpopexy (abdominal or<br />
laparoscopic) with mesh.<br />
See recommendation 1.8.6 for<br />
specific guidance on the use of mesh in<br />
prolapse surgery.<br />
1.8.17 If a synthetic polypropylene mesh<br />
is inserted, the details of the procedure<br />
and its subsequent short- and longterm<br />
outcomes must be collected in a<br />
national registry (see collecting data on<br />
surgery and surgical complications in this<br />
guideline).<br />
Colpocleisis for vault or uterine<br />
prolapse<br />
1.8.18 Consider colpocleisis for women<br />
with vault or uterine prolapse who do<br />
not intend to have penetrative vaginal<br />
sex and who have a physical condition<br />
that <strong>may</strong> put them at increased<br />
risk of operative and postoperative<br />
complications.<br />
Surgery for anterior prolapse<br />
1.8.19 Discuss the options for treatment<br />
(see recommendation 1.8.2), including<br />
non-surgical and surgical options, with<br />
women who have anterior prolapse.<br />
1.8.20 Offer anterior repair without mesh<br />
to women with anterior vaginal wall<br />
prolapse.<br />
1.8.21 Consider synthetic polypropylene<br />
or biological mesh insertion for women<br />
with recurrent anterior vaginal wall<br />
prolapse only after:<br />
• regional MDT review and<br />
• discussion with the woman about<br />
the risks of mesh insertion (see<br />
recommendation 1.8.2)<br />
and if:<br />
• apical support is adequate or<br />
• an abdominal approach is<br />
contraindicated.<br />
See recommendation 1.8.6 for<br />
specific guidance on the use of mesh in<br />
prolapse surgery.<br />
1.8.22 If a synthetic polypropylene or<br />
biological mesh is inserted, the details<br />
of the procedure and its subsequent<br />
short- and long-term outcomes must<br />
be collected in a national registry (see<br />
collecting data on surgery and surgical<br />
complications in this guideline).<br />
Surgery for posterior prolapse<br />
1.8.23 Offer posterior vaginal repair<br />
without mesh to women with a posterior<br />
vaginal wall prolapse.<br />
Follow-up after surgery<br />
1.8.24 Offer women a review 6 months<br />
after surgery for pelvic organ prolapse.<br />
May 2019 / FUTURE MEDICINE / 83
Ensure that the review includes a vaginal<br />
examination and, if mesh was used,<br />
check for mesh exposure.<br />
1.8.25 Providers should ensure that<br />
women who have had surgery for pelvic<br />
organ prolapse have access to further<br />
referral if they have recurrent symptoms<br />
or suspected complications. See also<br />
assessing complications associated with<br />
mesh surgery in this guideline.<br />
1.9 Surgery for women with both<br />
stress urinary incontinence and<br />
pelvic organ prolapse<br />
1.9.1 Consider concurrent surgery for<br />
stress urinary incontinence and pelvic<br />
organ prolapse in women with anterior<br />
and/or apical prolapse and stress urinary<br />
incontinence.<br />
1.9.2 When considering concurrent<br />
surgery for stress urinary incontinence<br />
and pelvic organ prolapse, discuss<br />
the options for treatment (see<br />
recommendations 1.5.1 and 1.8.2) and<br />
explain to the woman:<br />
• that there is uncertainty about<br />
whether the combined procedure is<br />
effective for treating stress urinary<br />
incontinence beyond 1 year, and that<br />
stress urinary incontinence might<br />
persist despite surgery<br />
• the risk of complications related<br />
to having surgery for stress urinary<br />
incontinence at the same time as<br />
prolapse surgery compared with the<br />
risk of complications related to having<br />
sequential surgery.<br />
1.10 Assessing complications<br />
associated with mesh surgery<br />
1.10.1 For women who report newonset<br />
symptoms after having mesh<br />
surgery for urinary incontinence or pelvic<br />
organ prolapse, evaluate whether the<br />
symptoms might be caused by a meshrelated<br />
complication. These symptoms<br />
could include:<br />
• pain or sensory change in the back,<br />
abdomen, vagina, pelvis, leg, groin or<br />
perineum that is:<br />
• either unprovoked, or provoked<br />
by movement or sexual activity and<br />
• either generalised, or in the<br />
distribution of a specific nerve, such as<br />
the obturator nerve<br />
• vaginal problems including<br />
discharge, bleeding, painful sexual<br />
intercourse, or penile trauma or pain in<br />
sexual partners<br />
• urinary problems including recurrent<br />
infection, incontinence, retention, or<br />
difficulty or pain during voiding<br />
• bowel problems including difficulty<br />
or pain on defaecation, faecal<br />
incontinence, rectal bleeding or<br />
passage of mucus<br />
• symptoms of infection, either alone<br />
or in combination with any of the<br />
symptoms outlined above.<br />
1.10.2 Refer women with a suspected<br />
mesh-related complication to a<br />
SURGERY TO REMOVE<br />
MESH CAN HAVE<br />
SIGNIFICANT COMPLICATIONS<br />
INCLUDING ORGAN INJURY,<br />
WORSENING PAIN<br />
urogynaecologist, urologist or colorectal<br />
surgeon for specialist assessment.<br />
1.10.3 For women who are referred for<br />
specialist evaluation of a suspected<br />
mesh complication:<br />
• take a history of all past surgical<br />
procedures for prolapse or<br />
incontinence using mesh, including the<br />
dates, type of mesh and site of mesh<br />
placement and the relationship of the<br />
symptoms to the surgical procedure(s)<br />
• consider using a validated pelvic<br />
floor symptom questionnaire and a<br />
pain questionnaire to aid assessment<br />
and decision making<br />
• perform a vaginal examination to:<br />
• assess whether mesh is palpable,<br />
exposed or extruded<br />
• localise pain and its anatomical<br />
relationship to mesh<br />
• consider performing a rectal<br />
examination, if indicated, to assess for<br />
the presence of mesh perforation or<br />
fistula<br />
• consider performing a neurological<br />
assessment to assess the distribution<br />
of pain, if present, sensory alteration or<br />
muscle weakness.<br />
1.10.4 For women with a confirmed<br />
mesh-related complication or<br />
unexplained symptoms after a mesh<br />
procedure:<br />
• refer to a consultant at a regional<br />
centre specialising in the diagnosis<br />
and management of mesh-related<br />
complications or<br />
• if the woman has a vaginal exposure<br />
of mesh that is smaller than 1 cm 2<br />
and no other symptoms, follow<br />
recommendations 1.11.3 and 1.11.4 in<br />
this guideline.<br />
1.10.5 The responsible consultant should<br />
develop an individualised investigation<br />
plan for each woman with suspected or<br />
confirmed mesh-related complications,<br />
involving other members of the regional<br />
MDT if needed, and use table 1 in this<br />
guideline to inform decisions on possible<br />
investigations.<br />
1.10.6 The responsible consultant must<br />
ensure that details of any confirmed<br />
mesh-related complications are:<br />
• recorded in a national registry (see<br />
the section on collecting data on<br />
surgery and surgical complications in<br />
this guideline) and<br />
• reported to the Medicines and<br />
Healthcare products Regulatory<br />
Agency (MHRA).<br />
1.11 Managing complications<br />
associated with mesh surgery<br />
General considerations before<br />
removing mesh<br />
1.11.1 If a woman who has had a mesh<br />
procedure to treat urinary incontinence<br />
or pelvic organ prolapse is thinking<br />
about having the mesh removed, discuss<br />
the decision with her and with a regional<br />
MDT.<br />
1.11.2 When discussing surgery to<br />
remove mesh, explain to the woman<br />
that:<br />
• there is limited evidence on the<br />
benefits of partial or complete removal<br />
compared with no mesh removal<br />
• surgery to remove mesh can have<br />
significant complications including<br />
organ injury, worsening pain, and<br />
84 / FUTURE MEDICINE / May 2019
INDIVIDUALISED INVESTIGATION PLANS<br />
Investigation Type of mesh Indications Benefits and risks<br />
Examination under<br />
anaesthesia<br />
All types of mesh.<br />
Pain or suspected:<br />
• vaginal or rectal exposure or<br />
extrusion<br />
• sinus tract, urinary or bowel fistula.<br />
Cystourethroscopy All types of mesh. Suspected:<br />
• urethral perforation<br />
• bladder perforation<br />
• fistula<br />
• calculus on suture or mesh material.<br />
Sigmoidoscopy<br />
Laparoscopy<br />
MRI, protocolled and<br />
reported by a clinician<br />
with experience in<br />
interpreting mesh<br />
complications<br />
Ultrasound scan<br />
(transperineal,<br />
transvaginal or<br />
translabial, or 3D),<br />
performed and<br />
reported by a clinician<br />
with experience in<br />
interpreting mesh<br />
complications<br />
CT<br />
Fluoroscopic studies<br />
(cystography or contrast<br />
enema)*<br />
Urinary flow studies<br />
and post-void residual<br />
volume assessment or<br />
cystometry<br />
Neurophysiology,<br />
including nerve<br />
conduction studies<br />
Abdominally,<br />
laparoscopically or<br />
vaginally placed<br />
mesh for pelvic organ<br />
prolapse.<br />
Abdominally or<br />
laparoscopically placed<br />
mesh for pelvic organ<br />
prolapse.<br />
All types of mesh.<br />
Vaginally placed mesh<br />
to treat incontinence.<br />
All types of mesh,<br />
although CT is not<br />
commonly used to<br />
show implanted<br />
material.<br />
Suspected bowel perforation by<br />
mesh.<br />
• Pain.<br />
• Suspected bowel entrapment<br />
around mesh.<br />
• Suspected adhesions secondary to<br />
mesh placement.<br />
Suspected mesh infection.<br />
Anatomical mapping of suspected<br />
fistula.<br />
Anatomical mapping and mesh<br />
localisation to guide further surgery.<br />
Back pain following abdominal mesh<br />
placement with mesh attachment to<br />
sacral promontory.<br />
Identification of discitis or<br />
osteomyelitis.<br />
Pain.<br />
Voiding dysfunction.<br />
Suspected infection.<br />
Suspected urethral mesh perforation.<br />
Anatomical mapping to guide excision<br />
surgery.<br />
Suspected:<br />
urinary tract injury<br />
bowel injury<br />
bowel obstruction.<br />
Benefits<br />
Allows diagnosis when not revealed by awake<br />
examination or when an awake, examination<br />
is not tolerated.<br />
Risks<br />
Anaesthetic risk.<br />
Benefits<br />
• Allows diagnosis by direct visualisation.<br />
• Aids management planning.<br />
Risks<br />
Anaesthetic risk and risk of urinary tract<br />
infection.<br />
Benefits<br />
• Allows diagnosis by direct visualisation.<br />
• Aids management planning.<br />
Risks<br />
• Anaesthetic risk if carried out under<br />
anaesthesia.<br />
• Risk of bowel perforation.<br />
Benefits<br />
• Allows diagnosis by direct visualisation.<br />
• Aids management planning.<br />
Risks<br />
• Anaesthetic risk.<br />
• Risks of laparoscopy, including bowel injury.<br />
Benefits<br />
Shows implanted material and complications<br />
nearby.<br />
Shows location of mesh in relation to the<br />
vaginal wall and sacrum.<br />
Risks<br />
Generally regarded as safe, with a low risk of<br />
short- and long-term harms. Risk of contrast<br />
media injection.<br />
Benefits<br />
Shows implanted material and local<br />
complications.<br />
Identifies mid-urethral slings.<br />
Shows location of mesh in relation to the<br />
vaginal wall and urethra.<br />
Risks<br />
Discomfort.<br />
Benefits<br />
May be useful in assessing for urinary fistulae<br />
or bowel injury.<br />
Risks<br />
Potential radiation-related harms and risk of<br />
contrast media injection.<br />
All types of mesh. Suspected urinary or bowel fistula. Benefits<br />
Aids management planning.<br />
Risks<br />
Potential radiation-related harms.<br />
All types of mesh.<br />
Voiding dysfunction.<br />
Urinary incontinence.<br />
Benefits<br />
Aids management planning.<br />
Risks<br />
Urinary tract infection and radiation risks if<br />
fluoroscopy is used.<br />
All types of mesh. Suspected nerve injury. Benefits<br />
Allows diagnosis of impaired nerve function.<br />
Risks<br />
Nerve conduction studies are difficult to<br />
perform and can induce more pain.<br />
* Perform with water-soluble contrast media. Fluoroscopic studies and CT <strong>may</strong> be used according to local preference and expertise.<br />
May 2019 / FUTURE MEDICINE / 85
urinary, bowel and sexual dysfunction<br />
• it is not certain that removing the<br />
mesh will relieve symptoms<br />
• it might not be possible to remove<br />
all of the mesh<br />
• removing only part of the mesh<br />
might be just as effective at improving<br />
symptoms as removing all of it<br />
• urinary incontinence or prolapse<br />
can recur after the mesh has been<br />
removed.<br />
Managing vaginal complications<br />
1.11.3 Discuss non-surgical treatment<br />
with topical oestrogen cream with<br />
women who have a single area of<br />
vaginal mesh exposure that is smaller<br />
than 1 cm 2 .<br />
1.11.4 Offer a follow‐up appointment<br />
within 3 months to women with vaginal<br />
mesh exposure who choose treatment<br />
with topical oestrogen cream.<br />
1.11.5 Consider partial or complete<br />
surgical removal of the vaginal portion of<br />
mesh for women:<br />
• who do not wish to have treatment<br />
with topical oestrogen or<br />
• if the area of vaginal mesh sling<br />
exposure is 1 cm 2 or larger or<br />
• if there is vaginal mesh extrusion or<br />
• if there has been no response to<br />
non-surgical treatment after a period<br />
of 3 months.<br />
1.11.6 Offer imaging and further<br />
treatment to women who have signs<br />
of infection in addition to vaginal mesh<br />
exposure or extrusion.<br />
1.11.7 Discuss with women who have<br />
vaginal complications after mesh sling<br />
surgery for stress urinary incontinence<br />
that:<br />
• complete removal of the vaginal<br />
portion of mesh sling is associated<br />
with a greater risk of recurrence of<br />
stress urinary incontinence than partial<br />
removal<br />
• partial removal is associated with<br />
a higher rate of further mesh sling<br />
extrusion<br />
• complete removal might not be<br />
possible.<br />
1.11.8 Explain to women who have<br />
vaginal complications after vaginally<br />
placed mesh for pelvic organ<br />
prolapse that:<br />
• complete removal might not be<br />
possible<br />
• complete removal has a higher risk<br />
of urinary tract or bowel injury than<br />
partial removal<br />
• there <strong>may</strong> be a risk of recurrent<br />
prolapse.<br />
1.11.9 Explain to women who have<br />
vaginal complications after abdominally<br />
placed mesh for pelvic organ prolapse<br />
that:<br />
• removal is associated with a risk of<br />
urinary tract and bowel injury<br />
• there is a risk of recurrent prolapse<br />
• they might need abdominal surgery<br />
to remove the mesh<br />
• complete removal might not be<br />
possible.<br />
1.11.10 For women who have pain or<br />
painful sexual intercourse suspected to<br />
be related to previous mesh surgery:<br />
• if specialist assessment indicates<br />
a mesh-related complication, seek<br />
advice from a regional MDT<br />
• if assessment and investigation do<br />
not show a mesh abnormality such<br />
as vaginal extrusion or exposure, or<br />
an infection, consider non-surgical<br />
treatments such as pain management,<br />
vaginal oestrogen, dilators, counselling<br />
(including psychosexual counselling)<br />
and physiotherapy<br />
• if pain does not respond to initial<br />
management, seek advice from a<br />
regional MDT.<br />
Managing urinary complications<br />
1.11.11 Refer women who have mesh<br />
perforating the lower urinary tract to a<br />
centre for mesh complications for further<br />
assessment or management. 1.11.12<br />
For women with urinary symptoms<br />
after mesh surgery for stress urinary<br />
incontinence or pelvic organ prolapse<br />
who are considering mesh removal<br />
surgery, explain that:<br />
• urinary symptoms might not improve<br />
and new symptoms might occur after<br />
complete or partial removal of the<br />
mesh<br />
• stress urinary incontinence might<br />
recur after mesh removal, and the<br />
risk of this happening is higher with<br />
complete than with partial mesh<br />
removal<br />
• complete removal of the mesh might<br />
not be possible<br />
• further treatment might be needed<br />
for mesh complications, or recurrent or<br />
persistent urinary symptoms<br />
• there is a risk of adverse events such<br />
as urinary tract fistula.<br />
1.11.13 Discuss division of mesh sling with<br />
women who have voiding difficulty after<br />
mesh sling surgery.<br />
1.11.14 Refer women considering excision<br />
of mesh sling for persistent voiding<br />
dysfunction to a centre specialising<br />
in the diagnosis and management<br />
of mesh-related complications for<br />
assessment and management.<br />
1.11.15 For women considering surgery<br />
to alleviate voiding symptoms caused by<br />
mesh surgery, explain that:<br />
• the risk of recurrent stress urinary<br />
incontinence is higher after mesh<br />
excision than mesh division<br />
• further surgery might be needed.<br />
Managing bowel symptoms<br />
1.11.16 For women who present with<br />
functional bowel disorders after mesh<br />
surgery for pelvic organ prolapse, follow<br />
the recommendations in the NICE<br />
guideline on faecal incontinence in adults<br />
for women with faecal incontinence or<br />
locally agreed protocols for women with<br />
obstructed defecation.<br />
1.11.17 For women with bowel<br />
complications that are directly related<br />
to mesh placement, such as erosion,<br />
stricture or fistula, discuss treatment<br />
with a regional MDT that has expertise<br />
in complex pelvic floor dysfunction<br />
and mesh-related problems. Use this<br />
discussion to formulate an individualised<br />
treatment plan with the woman.<br />
1.11.18 Explain to women with bowel<br />
complications directly related to mesh<br />
placement that:<br />
• complete removal might not be<br />
possible<br />
• bowel symptoms might persist or<br />
recur after mesh removal<br />
• they might need a temporary or<br />
permanent stoma after mesh<br />
removal.<br />
86 / FUTURE MEDICINE / May 2019
Keep updated with the brave new world of medicine
CUTTING-EDGE ADVANCES IN<br />
INTERVENTIONAL TECHNOLOGY<br />
ARE TAKING CARDIOVASCULAR<br />
MEDICINE BY STORM<br />
INHERITED CVD:<br />
BIGGEST KILLER<br />
GETS OFF SCOT-FREE<br />
MBBS CURRICULUM<br />
REVAMPED<br />
HEART RHYTHM<br />
DISORDERS<br />
MIMIC EPILEPSY<br />
NON-FUNCTIONAL<br />
PLATELETS<br />
DNBs SEEK<br />
EQUIVALENCE<br />
WITH MD<br />
EXCLUDING<br />
EXCLUSIONS<br />
₹ 250.00<br />
VOL 5 | ISSUE 5<br />
PAGES 100<br />
CARDIOGENETICS CASE REPORT EDUCATION ONCOLOGY<br />
₹ 250.00<br />
VOL 5 | ISSUE 8<br />
PAGES 100<br />
INNOVATIVE THERAPEUTIC STRATEGIES<br />
TO REIN IN THE HIV CONTAGION<br />
EDUCATION CASE REPORT HEALTH INSURANCE POLICY<br />
SEPTEMBER 2018<br />
FUTUREMEDICINEINDIA.COM<br />
GINGIVO-BUCCAL<br />
CANCERS ARE<br />
DIFFERENT<br />
DECEMBER 2018<br />
FUTUREMEDICINEINDIA.COM<br />
BEHIND HIGH<br />
DRUG PRICES<br />
COMBO-DRUGS<br />
ON THEIR<br />
WAY OUT?<br />
AI, DEEP LEARNING AND BIG DATA USHER IN<br />
A NEW ERA OF MEDICAL IMAGING<br />
ADVANCED<br />
ORTHO IMAGING<br />
MICRODELETION:<br />
LOST IN<br />
TRANSLATION<br />
CONSUMER BILL:<br />
DOCTORS<br />
DISMAYED<br />
ORAL CANCER<br />
STAGING BY<br />
DEPTH OF INVASION<br />
IR: SPEARHEAD<br />
OF LESS-INVASIVE<br />
MEDICINE?<br />
₹ 250.00<br />
VOL 5 | ISSUE 6<br />
PAGES 100<br />
₹ 250.00<br />
VOL 5 | ISSUE 9<br />
PAGES 100<br />
ORTHOPAEDICS POLICY SPECIALTIES CASE REPORT<br />
OCTOBER 2018<br />
FUTUREMEDICINEINDIA.COM<br />
NON-INVASIVE PRENATAL<br />
SCREENING ENTERS<br />
REGULATORY DIAGNOSTICS HEAD & NECK CANCER CASE REPORT<br />
EXCITING FRONTIERS<br />
HOW SHE<br />
REGAINED<br />
HER LOST TASTE<br />
JANUARY 2019<br />
FUTUREMEDICINEINDIA.COM<br />
FACTS ON<br />
FANCONI<br />
IMMUNOTHERAPY FOR<br />
WILL CHECKPOINT BLOCKERS ALTER<br />
WELL-ENTRENCHED TREATMENT ALGORITHMS?<br />
ABERNETHY -<br />
AN UNUSUAL<br />
SUSPECT<br />
A HARROWING<br />
SWALLOW<br />
TACKLING<br />
EMERGING<br />
PATHOGENS<br />
INTERNSHIP<br />
FOR FOREIGN<br />
GRADUATES?<br />
MCI IN DIRE<br />
STRAITS<br />
FLUID DIAGNOSIS<br />
FOR DEMENTIA<br />
₹ 250.00<br />
VOL 5 | ISSUE 7<br />
PAGES 100<br />
CASE REPORT DRUG RESISTANCE REGULATORY COPD<br />
₹ 250.00<br />
VOL 5 | ISSUE 10<br />
PAGES 100<br />
CASE REPORT EDUCATION RESEARCH ONCO SURGERY<br />
NOVEMBER 2018<br />
FUTUREMEDICINEINDIA.COM<br />
EBV INTERVENTION<br />
IN EMPHYSEMA<br />
FEBRUARY 2019<br />
FUTUREMEDICINEINDIA.COM<br />
NOVEL SURGICAL<br />
OPTIONS FOR<br />
BREAST CANCER<br />
Hello,<br />
Greetings from Future Medicine!<br />
Future Medicine, India’s first medical science news<br />
magazine published by NextGen Science Media for the<br />
medical fraternity in India. This is specifically aimed<br />
at updating the community with the latest scientific<br />
developments and trends from across the globe.<br />
Future Medicine is a pioneering concept in India to<br />
seamlessly connect the country’s medical community<br />
with the brave new world of evolving science and<br />
medicine.<br />
We do this through quick and comprehensive updates<br />
on new discoveries, technology breakthroughs and case<br />
reports among others from across the world in a simple<br />
and interesting way. This monthly science news<br />
magazine also provides researchers, medical<br />
authors and thinking minds in public healthcare<br />
a platform to discuss their views and works for<br />
the benefit of thecommunity and the public at large.<br />
SPECIAL SUBSCRIPTION<br />
OFFER<br />
FOR MEDICAL STUDENTS<br />
1 Year<br />
(12 Issues)<br />
Cover Price<br />
Rs. 3000/-<br />
Special Offer for<br />
Medical Students<br />
Rs. 1800/-<br />
2 Year<br />
(24 Issues)<br />
Cover Price<br />
Rs. 6000/-<br />
Special Offer for<br />
Medical Students<br />
Rs. 3000/-<br />
40 50<br />
TRANSCATHETER<br />
TRANSFORMATION<br />
KNOWING<br />
THE UNBORN<br />
LUNG<br />
CANCER<br />
ENCOUNTERING<br />
AN ELUSIVE<br />
VIRUS<br />
THE BRAVE<br />
NEW WORLD<br />
OF IMAGING<br />
STARTING TO<br />
FORGET<br />
THE SPECTRE OF ALZHEIMER’S LOOMS LARGE OVER<br />
INDIA’S FAST-EXPANDING POPULATION OF THE AGED<br />
Fill complete details and send it along with the cheque/DD in favour of ‘NEXTGEN SCIENCE MEDIA (P.) LTD.’ to Future Medicine, 7th floor,<br />
Wework Zenia, Zenia Building, Hiranandani Business Park, Off. Ghodbunder Road, Thane, Mumbai, MH- 400 607<br />
or Scan the filled form and mail it to subscribe@futuremedicineindia.com<br />
• For NEFT/RTGS : Account No. 50200032001372, IFSC:- HDFC0000684 Name: NextGen Science Media Pvt Ltd, HDFC Bank Ltd<br />
• For more details call - 9594 625 231 or mail - subscribe@futuremedicineindia.com<br />
Please send me my subscription of FUTURE MEDICINE for (Select your plan)<br />
One year Rs. 1,800/- Two years Rs. 3,000/-<br />
NAME<br />
ADDRESS<br />
COLLEGE<br />
POSTAL CODE<br />
E-MAIL<br />
PHONE
events<br />
7th Intl meet on rare diseases<br />
puts India in focus<br />
Experts highlight the importance of investigating the genetic disorders<br />
which are largely left undiagnosed<br />
India is home to an estimated<br />
70 million people affected by<br />
undiagnosed or rare diseases. Of<br />
these, most are carriers of some<br />
genetic disorder or the other. With<br />
the advent of the latest sequencing,<br />
newborn screening and other<br />
technologies, a few genetic disorders<br />
such as Down’s syndrome, beta<br />
thalassemia and sickle cell anaemia<br />
etc. are being well tracked and have<br />
been found to be on the rise. Recent<br />
estimates show that at least 21,400<br />
children are born every year in India<br />
with Down’s syndrome and another<br />
9,000 to 10,000 children with<br />
thalassemia. While these diseases<br />
remain inadequately addressed as the<br />
patients often do not recognize the<br />
symptoms, the country’s problems<br />
THE MESSAGE AT THE 7TH<br />
INTERNATIONAL CONFERENCE<br />
ON ‘RARE AND UNDIAGNOSED<br />
DISEASES: ADDRESSING<br />
PATIENT NEEDS IN INDIA’ WAS<br />
LOUD AND CLEAR<br />
with rare and undiagnosed diseases<br />
continue to be worrying as there aren’t<br />
enough trained clinicians to detect<br />
and diagnose them. So, it is anyone’s<br />
guess as to how bad the scenario is<br />
in a country of 1.2 billion population<br />
with widely varied communities and<br />
subgroups carrying different genetic<br />
pro<strong>file</strong>s and, potentially, an array of<br />
known and unknown genetic mutations.<br />
Not surprisingly, every year,<br />
thousands of men, women and children<br />
face uncertainty when healthcare<br />
providers are unable to discover the<br />
cause for their symptoms. In India, with<br />
its low awareness of such diseases,<br />
a large number of patients are left<br />
with no treatment and succumb to<br />
their condition. To make it worse, drug<br />
researchers and the industry often<br />
neglect such diseases even if they are<br />
diagnosed, as the market size for such<br />
rare disorders are either not estimated<br />
or comparatively much smaller.<br />
The message at the 7th International<br />
Conference on ‘Rare and Undiagnosed<br />
Diseases: Addressing Patient Needs<br />
90 / FUTURE MEDICINE / May 2019
in India’ was loud and clear — to<br />
make these relevant. The three-day<br />
event organised in Delhi in April<br />
by Undiagnosed Disease Network<br />
International (UDNI), Institute of Medical<br />
Genetics & Genomics at Sir Ganga<br />
Ram Hospital, Wilhelm Foundation,<br />
Organisation of Rare Diseases India<br />
(ORDI) and Institute of Genomics<br />
& Integrative Biology (IGIB) of CSIR<br />
called for the best and most effective<br />
collaborative efforts both nationally and<br />
internationally to share such experiences<br />
and find the way forward to solve and<br />
help patients with such conditions.<br />
“In India, the scenario is more<br />
complex as the volume is enormous and<br />
it has many communities and different<br />
subgroups. Because we don’t have<br />
the frequency of variance of each of<br />
the subgroups, it becomes much more<br />
difficult to diagnose new diseases,” said<br />
William A Gahl from National Human<br />
Genome Research Institute at the NIH,<br />
US.<br />
Dr Gahl, who is also the Director at<br />
NIH Undiagnosed Diseases Programme,<br />
added that most of the rare and<br />
undiagnosed diseases have got some<br />
or other link with the genetic base. “We<br />
have seen at the NIH itself about 1,200<br />
to 1,250 patients and made some 320<br />
diagnosis. We have discovered about 25<br />
new diseases in the last ten years where<br />
a gene is associated with a particular<br />
phenotype of presentation.”<br />
According to Dr I C Verma, advisor<br />
and key member of the scientific<br />
committee of the Conference, the<br />
main message of the 7th International<br />
Conference is that it is worth<br />
investigating the cause of rare and<br />
undiagnosed disorders, as this has<br />
been made easier by the application<br />
of massively parallel sequencing<br />
technology.<br />
“Knowing the diagnosis, one<br />
proceeds to finding a treatment, which<br />
is the main concern of the patient and<br />
the family. Not knowing the diagnosis is<br />
very frustrating,” quipped Dr Verma.<br />
“Contrary to usual thinking, rare and<br />
undiagnosed disorders affect a fairly<br />
large number of people in India,” added<br />
In India, the scenario is more<br />
complex as the volume is<br />
enormous and it has many<br />
communities and different<br />
subgroups.<br />
Dr William A Gahl<br />
Director, NIH Undiagnosed Diseases<br />
Programme<br />
Verma, Advisor & Senior Consultant and<br />
Professor of Medical Genetics at Institute<br />
of Medical Genetics and Genomics, Sir<br />
Ganga Ram Hospital, while talking to<br />
Future Medicine in a post-conference<br />
interview.<br />
Attended by some 370 registered<br />
delegates, including practicing<br />
clinicians, students and researchers,<br />
the Conference was immensely rich in<br />
content with about 60 speakers from<br />
across the world sharing their case<br />
studies, experiments, ideas and vision.<br />
“One of the key objectives<br />
ofconferring here was to communicate<br />
to our Indian colleagues and medical<br />
students about patients suffering from<br />
undiagnosed diseases,” said Dr Ratna<br />
Dua Puri, organizing chairperson of the<br />
Conference, in an interview with Future<br />
Medicine.<br />
She added: “With a lot of the latest<br />
May 2019 / FUTURE MEDICINE / 91
sequencing facilities now available<br />
in the country and a fair number of<br />
clinical geneticists able to do deep<br />
phenotyping, we are able to help many<br />
patients. But, despite all these, there<br />
are still several cohorts with genetic<br />
disorders left undiagnosed. So, it is<br />
important to collaborate with colleagues<br />
and link the work done here as well as<br />
at other places and take the initiative<br />
as a community. This was the other<br />
main aim of this conference held in<br />
partnership with Undiagnosed Disease<br />
Network International.”<br />
This kind of conferences are also<br />
a wake-up call for countries like India,<br />
where genetic and metabolic disorders<br />
are usually diagnosed with poor<br />
outcomes.<br />
“On this front, the major challenges<br />
in India at present include the small<br />
number of trained clinicians specialising<br />
in diagnosing and counselling genetic<br />
disorders, the few state-funded<br />
diagnostic and research laboratories, a<br />
lack of funding for genetic diagnostic<br />
tests and less accessibility for therapies<br />
and other interventions,” said Dr<br />
Meenakshi Bhat, Senior Consultant in<br />
Clinical Genetics at Centre for Human<br />
Genetics at Indira Gandhi Institute of<br />
Child Health, Bangalore.<br />
According to Dr Seema Kapoor,<br />
in-charge of Genetics and Metabolism<br />
Division of Maulana Azad Medical<br />
College, New Delhi, though India has<br />
achieved significant milestones towards<br />
tracking genetic disorders through<br />
It is worth investigating the<br />
cause of rare and undiagnosed<br />
disorders, as this has been<br />
made easier by the application<br />
of massively parallel sequencing<br />
technology.<br />
Dr I C Verma<br />
Advisor and key member,<br />
Scientific Committee<br />
several government funded initiatives,<br />
including newborn screening for many<br />
congenital diseases since 2013, the<br />
challenges that still invite discussion<br />
are the feasibility of coverage in less<br />
developed areas, hilly terrains and home<br />
deliveries.<br />
“Besides, the availability of good<br />
counsellors and a well-integrated followup<br />
system needs to be developed,” she<br />
said.<br />
However, there is light at the end<br />
of the tunnel. Among others, one of<br />
the most recent collaborative research<br />
programmes—The Genomics for<br />
Understanding Rare Diseases: India<br />
Alliance Network (GUaRDIAN) initiated<br />
by the IGIB, has undertaken whole<br />
exome and genome sequencing for<br />
identification of variants and genes<br />
implicated in rare genetic diseases.<br />
“Using Mitochondrial rare genetic<br />
disease examples, we will be able<br />
to share our experiences from the<br />
GUaRDIAN consortium where we<br />
apply genome sequencing followed by<br />
computational analysis and zebrafish<br />
disease modelling to solve several<br />
cases of undiagnosed diseases,” said Dr<br />
Sridhar Sivasubbu, Principal Scientist at<br />
CSIR-IGIB.<br />
No doubt, only collaborative models<br />
will work in such a complex task of<br />
diagnosing unknown diseases to find<br />
treatments for them. Dr Gahl concludes<br />
that since the first meeting of the UDNI<br />
in Rome in 2014, there have been six<br />
international meetings, culminating in<br />
the 2019 conference in Delhi. In the<br />
past five years, over a dozen countries<br />
have established Undiagnosed Disease<br />
Programmes and the Network has<br />
created several common platforms to<br />
share experiences, information and<br />
views. The main focus of the Network<br />
involves sharing of genotypic and<br />
phenotypic data and best practices.<br />
And this, amongst others, can<br />
ultimately help advance the diagnosis<br />
and care of rare and undiagnosed<br />
diseases.<br />
92 / FUTURE MEDICINE / May 2019
events<br />
Cahocon 2019 highlights need for<br />
patient safety<br />
5th <strong>edition</strong> of CAHO conference explores ways and means to minimize medical errors<br />
DR SUMIT GHOSHAL<br />
The quality of service provided<br />
by healthcare providers and<br />
the related issue of patient<br />
safety is a major concern of hospital<br />
managements around the country.<br />
Mistakes in the treatment and<br />
healthcare services can not only affect<br />
the well-being of individual patients<br />
and their families but also create a trust<br />
deficit between the healthcare provider<br />
and the service consumers.<br />
To discuss these questions and<br />
to seek ways and means to minimize<br />
errors at various levels, senior<br />
management executives of leading<br />
Indian hospitals got together in<br />
Mumbai at the 5th national conference<br />
of CAHO (Consortium of Accredited<br />
Healthcare Organizations). These are<br />
hospitals and healthcare organisations<br />
that have obtained an accreditation<br />
from the NABH (National Board of<br />
Accreditation of Hospitals), a quasiofficial<br />
certification body functioning<br />
under the aegis of the Quality Council<br />
of India (QCI).<br />
The NABH, which was established<br />
in 2005-06, offers accreditation at<br />
two levels: An entry-level certificate<br />
and full-accreditation. The entry level<br />
certification was instituted along the<br />
way when NABH leaders realised that<br />
many hospitals were unable to meet<br />
With accreditation, though it<br />
has a cost attached to it, there<br />
is an element of discipline<br />
in the healthcare. This has<br />
resulted in patient safety and<br />
better treatment outcome<br />
undoubtedly.<br />
Dr Rajesndra Patankar<br />
Chief Operating Officer<br />
Nanavati Super Specialty Hospital<br />
94 / FUTURE MEDICINE / May 2019
the criteria for full-accreditation at the<br />
start.<br />
Discussing the healthcare scenario<br />
before the accreditation system was<br />
set up in India, Air Marshal (Dr) Pawan<br />
Kapoor, vice chairman, RUS Education<br />
and vice chancellor, Lincoln American<br />
University stated that awareness of<br />
quality issues in healthcare arose<br />
when Consumer Protection Act 1986<br />
was made applicable to doctors and<br />
hospitals. Till then, mistakes and<br />
mishaps involving clinicians were<br />
carefully hidden with the express<br />
purpose of protecting healthcare<br />
practitioners from being blamed for<br />
patient death or injury.<br />
At present, about 600 large and<br />
medium healthcare institutions, along<br />
with about 218 small hospitals, have<br />
been able to obtain the entry-level<br />
certification from NABH, Air Marshal<br />
Kapoor said. He also provided extensive<br />
statistical data on improvements<br />
recorded in accredited hospitals in<br />
terms of fewer mistakes, better patient<br />
outcomes and so on.<br />
A highlight of the recent CAHO<br />
conference was the launch of entry<br />
level certification by the National<br />
Accreditation Board for Laboratories<br />
(NABL), which is a sister organization<br />
of NABH. Currently, about 1,100<br />
pathology labs have obtained the full<br />
accreditation from NABL, which is<br />
a really small number compared with<br />
the total of about 50,000 to<br />
80,000 laboratories present inall<br />
over the country.<br />
The NABL has not only launched<br />
a number of schemes to enable small<br />
laboratories to join the accreditation<br />
system, but is also getting ready to<br />
include the hundreds of radiology and<br />
imaging laboratories under its ambit.<br />
Dwelling on the impact of<br />
The time has come to<br />
have an accountability<br />
on healthcare delivery<br />
standards and accredit<br />
healthcare organizations<br />
based on the quality of<br />
healthcare they provide to<br />
patients.<br />
Dr Aparna Jairam<br />
Founder & Director<br />
Asavalee Dr Aparna's Pathology<br />
Laboratory and Co-organising<br />
Secretary CAHOCon-2019<br />
errors in patient care, Dr Krishnan<br />
Sankaranayaranan, Patient Safety<br />
Officer, with Tawam Hospital in<br />
Abu Dhabi, pointed out that the<br />
healthcare workers responsible for a<br />
serious error wasare often impacted<br />
almost as severely as the patient<br />
concerned. In international literature,<br />
this is being described as “becoming<br />
the second victim”, the first victim<br />
of the mistake being the affected<br />
patient.<br />
Dr Krishnan emphasized that the<br />
second victim could suffer severe<br />
depression and even denial of the<br />
responsibility, and later self-doubt and<br />
a loss of self-confidence. There have<br />
been many instances where such a<br />
healthcare professional might move<br />
to a less stressful department in the<br />
hospital, or in extreme cases move<br />
away from the healthcare profession<br />
completely, he said.<br />
Dr Sanjay Oak, who has held a<br />
number of top positions in public<br />
health and education, including<br />
that of vice chancellor of D Y Patil<br />
University, Navi Mumbai, pointed out<br />
that there were several gaps in the<br />
way medical education was imparted<br />
in most medical colleges. He said<br />
there was an urgent need for modern<br />
teaching methods, including simulation<br />
modules, that could allow the students<br />
to appreciate both the structure<br />
and the functioning of the human<br />
body without endangering an actual<br />
human being.<br />
May 2019 / FUTURE MEDICINE / 95
calendar<br />
Upcoming conferences<br />
APR<br />
26-11<br />
MAY<br />
PHYSICAL THERAPY<br />
FM UQ: Functional Mobilization<br />
Upper Quadrant<br />
New Delhi<br />
2-5 NEPHROLOGY AND<br />
UROLOGY<br />
ApEx-Cedars Sinai: The 10th<br />
Annual Nephrology Board<br />
Review Course and Urology for<br />
Nephrologists Workshop<br />
Mumbai<br />
3-5 MED EQUIPMENT<br />
MEDIKO India (MEDIKA)<br />
Hyderabad<br />
6-10 ONCOLOGY<br />
Cardiff-Tata Medical Center FRCR<br />
2B Oncology Course<br />
Kolkata<br />
11 BIOTECHNOLOGY<br />
Innovative Research in<br />
Bioscience, Bioinformatics,<br />
Biomedical Engineering<br />
Cancer Biology and Applied<br />
Biotechnology<br />
Delhi<br />
23-24 CLINICAL GENETICS<br />
CRO/Sponsor Summit 2019<br />
Hyderabad<br />
24-26 EXPO<br />
Medical Expo India, Indore (MEI)<br />
Indore<br />
26-11 PHYSICAL THERAPY<br />
FM UQ: Functional Mobilization<br />
Upper Quadrant<br />
New Delhi<br />
28 CLINICAL TRIAL<br />
10th Annual Clinical Trials<br />
Summit (Clinical Trials Asia)<br />
Mumbai<br />
31-2 NEUROLOGY<br />
Asian And Oceanian Myology<br />
Center Meeting (AOMC Meeting)<br />
Mumbai<br />
JUNE<br />
7-9 GYNAECOLOGY<br />
Annual Conference of the Indian<br />
Association of Gynaecological<br />
Endoscopists<br />
Ahmedabad<br />
8-9 OPHTHALMOLOGY<br />
National Conference on<br />
Community Ophthalmology<br />
Chennai<br />
27-30 SONOGRAPHY<br />
Sono Summit<br />
Chennai<br />
28-30 EXPO<br />
India Med Expo (IME)<br />
Bengaluru<br />
JULY<br />
2-4 DERMATOLOGY<br />
Congress of Cosmetic<br />
Dermatology Society<br />
(COSDERMINDIA)<br />
Mumbai<br />
3-4 OPHTHALMOLOGY<br />
Ophthall (OPH)<br />
Hyderabad<br />
3-4 SURGERY<br />
World Congress & Summit on<br />
Surgery<br />
New Delhi<br />
3-5 OPHTHALMOLOGY<br />
India International Optical &<br />
Ophthalmology Expo (IIOO<br />
EXPO)<br />
Hyderabad<br />
9-11 CARDIOLOGY<br />
Echo Nagpur Summit<br />
Nagpur<br />
15-18 TRAUMA S URGERY<br />
Traumacon<br />
Mumbai<br />
23-25 HEMATOLOGY<br />
Hope Asia 2019 Kolkata<br />
Kolkata<br />
AUGUST<br />
3-4 OPHTHALMOLOGY<br />
Ophthall (OPH)<br />
Hyderabad<br />
3-4 SURGERY<br />
World Congress & Summit on<br />
Surgery<br />
New Delhi<br />
3-5 OPHTHALMOLOGY<br />
India International Optical &<br />
Ophthalmology Expo (IIOO<br />
EXPO)<br />
Hyderabad<br />
9-11 CARDIOLOGY<br />
Echo Nagpur Summit<br />
Nagpur<br />
23-25 HEMATOLOGY<br />
Hope Asia 2019<br />
Kolkata<br />
SEPTEMBER<br />
5-8 GYNAECOLOGY<br />
33rd Annual Conference of AICC<br />
RCOG 2019<br />
Kolkata<br />
6-8 ONCOLOGY<br />
23rd Annual Conference of<br />
Pediatric Hematology Oncology<br />
Chapter (PHO)<br />
Varanasi<br />
6-8 CARDIOLOGY<br />
10th National Annual<br />
Conference of Indian Association<br />
of Clinical Cardiologists<br />
Conference (IACCCON 2019)<br />
Kochi<br />
10-11 PAEDIATRICS<br />
World Pediatrics Conference<br />
Panjim<br />
10-11 CARDIOLOGY<br />
World Heart and Cardiothoracic<br />
Surgery Conference (WHCS<br />
Heart Conference)<br />
Cavelossim<br />
10-11 PULMONARY C ARE<br />
Pulmonary, Thoracic and Critical<br />
Care Conference (PTCC)<br />
Goa<br />
13-14 ONCOLOGY<br />
Indo Oncology Summit<br />
(IOS-Bhubaneswar)<br />
Bhubaneswar<br />
13-15 PSYCHOLOGY<br />
Fortis Annual Psychology<br />
Conference<br />
Gurgaon<br />
20-22 SURGICAL O NCOLOGY<br />
National Conference of Indian<br />
Association of Surgical Oncology<br />
(NATCON IASO)<br />
Kolkata<br />
27-29 ARTHROSCOPY<br />
Conference of Indian<br />
Arthroscopy Society (IASCON)<br />
Indore<br />
27-29 CARDIOLOGY<br />
Global Cardio Diabetes Conclave<br />
(GCDC)<br />
Mumbai<br />
29-<br />
Oct 2<br />
30-<br />
Oct 2<br />
TRANSPLANT S URGERY<br />
CAST 2019 - 16th Congress<br />
of the Asian Society of<br />
Transplantation<br />
Delhi<br />
NGBT<br />
2019 Nextgen genomics biology,<br />
bioinformatics and technologies<br />
conference<br />
Mumbai<br />
The announced dates of the conferences <strong>may</strong> change<br />
96 / FUTURE MEDICINE / May 2019
AUGUST 2018/ FUTURE MEDICINE / 85
UNDERSTAND THE FUTURE NOW<br />
AND APPLY IT IN YOUR PRACTICE<br />
PROF DR I C VERMA<br />
Advisor & Senior Consultant, Institute of Medical Genetics, Sir Ganga Ram Hospital, New Delhi<br />
The age of genomic medicine is upon us and<br />
we are rapidly moving into the era of precision<br />
medicine. Young clinicians should spend time to<br />
understand this technology and learn to interpret the<br />
results.<br />
Catching this technology at an early age will also<br />
help them significantly contribute to the society<br />
by helping patients who suffer from rare and yet<br />
undiagnosed diseases.<br />
Addressing the medical needs of such patients is<br />
one of the key responsibilities of the advanced medical<br />
world of today. But, unfortunately, this hasn’t yet got<br />
due attention from the young and emerging clinicians<br />
here, unlike in the West.<br />
In the western world, the need for addressing<br />
rare and undiagnosed diseases, which are mostly<br />
connected with genetic causes, has got serious<br />
attention from the clinician community, especially the<br />
young who are keen to devote time for research. This<br />
is not only satisfying intellectually, but also translates<br />
into financial gains if a new therapy is discovered.<br />
Genetics and genomic studies have a lot more to<br />
do in order to address rare and undiagnosed diseases.<br />
But, sadly the current medical curriculum in India is<br />
not geared to prepare students to understand or apply<br />
genomics in healthcare.<br />
In the UK, they are carrying out genomic studies<br />
in 100,000 people at present, while in the US it<br />
is an extensive study in about 1 million people,<br />
to understand the interplay of genetics and the<br />
environment to cause diseases.<br />
Understanding this would lead to the development<br />
of novel and precise preventive, therapeutic and<br />
curative approaches. Moving a step ahead, the UK has<br />
already announced that genomics will henceforth be a<br />
part and parcel of national health services.<br />
The younger generation in this profession, if<br />
equipped with new and emerging technologies, can<br />
contribute to the critical needs of patients who have<br />
genetic as well as currently incurable diseases.<br />
Precision medicine — the concept of tailoring the<br />
medicine to the genetic as well as environmental<br />
attributes of the patient —is the future. Be prepared<br />
for it and apply it in your practice today.<br />
— As told to CH Unnikrishnan<br />
98 / FUTURE MEDICINE / May 2019
Sep 30 th - Oct 2 nd , 2019 - Mumbai, India<br />
REGISTRATIONS OPEN<br />
Early bird ends on<br />
15 th June, 2019<br />
Abstract submission<br />
ends on 1 st July, 2019<br />
NextGen Genomics, Biology, Bioinformatics and Technologies (NGBT) Conference is an international<br />
meeting organized by SciGenom Research Foundation (SGRF), a not-for-profit organization working to<br />
promote Science, Research and Education in India and rest of Asia.<br />
HIGHLIGHTS<br />
• Learn about cutting edge developments in<br />
genomics, biology, bioinformatics, drug<br />
discovery, plant and animal sciences<br />
• Network with scientists of global repute<br />
• Meet national and international genomics,<br />
biology and technology companies<br />
• Interact with leaders from drug industry<br />
• Explore collaboration opportunities<br />
• Scholarship opportunities for students to support<br />
their participation at the meeting<br />
KEY FOCUS AREA<br />
• Genomics technologies<br />
• Population genomics<br />
• Clinical/Medical genomics<br />
• NIPT/Liquid biopsy<br />
• Precision (personalized) medicine<br />
• Cancer genomics<br />
• CRISPR/CAS9<br />
• Gene editing<br />
• Signal transduction<br />
• Cancer immunology<br />
• Biomarkers<br />
• Drug discovery<br />
• Metagenomics<br />
• Plant genomics/sciences<br />
• Agriculture genomics/sciences<br />
• Veterinary genomics/sciences<br />
• Wildlife genomics/conservation<br />
100+<br />
Speakers<br />
Ms. Ms. Kamalika Das Das<br />
+91- 8374 27 4074<br />
800+<br />
Delegates<br />
ngbt2019@sgrf.org<br />
300+<br />
Posters<br />
www.sgrfconferences.org<br />
100+<br />
Scholarships & Awards
RNI Number KERENG/2012/44529