YSM Issue 90.5
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
FOCUS<br />
cell biology<br />
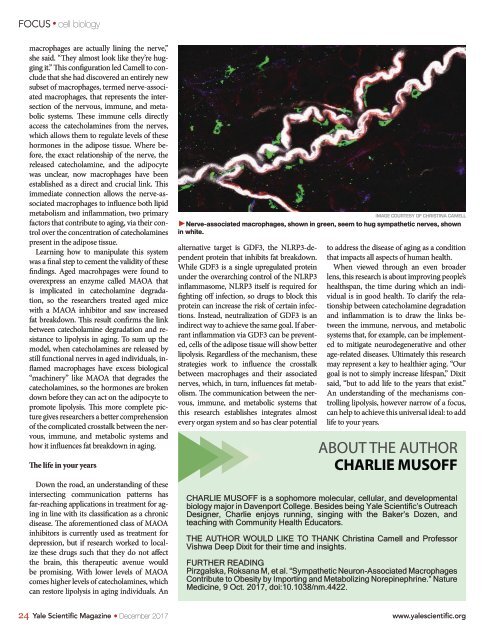
macrophages are actually lining the nerve,”<br />
she said. “They almost look like they’re hugging<br />
it.” This configuration led Camell to conclude<br />
that she had discovered an entirely new<br />
subset of macrophages, termed nerve-associated<br />
macrophages, that represents the intersection<br />
of the nervous, immune, and metabolic<br />
systems. These immune cells directly<br />
access the catecholamines from the nerves,<br />
which allows them to regulate levels of these<br />
hormones in the adipose tissue. Where before,<br />
the exact relationship of the nerve, the<br />
released catecholamine, and the adipocyte<br />
was unclear, now macrophages have been<br />
established as a direct and crucial link. This<br />
immediate connection allows the nerve-associated<br />
macrophages to influence both lipid<br />
metabolism and inflammation, two primary<br />
factors that contribute to aging, via their control<br />
over the concentration of catecholamines<br />
present in the adipose tissue.<br />
Learning how to manipulate this system<br />
was a final step to cement the validity of these<br />
findings. Aged macrohpages were found to<br />
overexpress an enzyme called MAOA that<br />
is implicated in catecholamine degradation,<br />
so the researchers treated aged mice<br />
with a MAOA inhibitor and saw increased<br />
fat breakdown. This result confirms the link<br />
between catecholamine degradation and resistance<br />
to lipolysis in aging. To sum up the<br />
model, when catecholamines are released by<br />
still functional nerves in aged individuals, inflamed<br />
macrophages have excess biological<br />
“machinery” like MAOA that degrades the<br />
catecholamines, so the hormones are broken<br />
down before they can act on the adipocyte to<br />
promote lipolysis. This more complete picture<br />
gives researchers a better comprehension<br />
of the complicated crosstalk between the nervous,<br />
immune, and metabolic systems and<br />
how it influences fat breakdown in aging.<br />
The life in your years<br />
Down the road, an understanding of these<br />
intersecting communication patterns has<br />
far-reaching applications in treatment for aging<br />
in line with its classification as a chronic<br />
disease. The aforementioned class of MAOA<br />
inhibitors is currently used as treatment for<br />
depression, but if research worked to localize<br />
these drugs such that they do not affect<br />
the brain, this therapeutic avenue would<br />
be promising. With lower levels of MAOA<br />
comes higher levels of catecholamines, which<br />
can restore lipolysis in aging individuals. An<br />
IMAGE COURTESY OF CHRISTINA CAMELL<br />
►Nerve-associated macrophages, shown in green, seem to hug sympathetic nerves, shown<br />
in white.<br />
alternative target is GDF3, the NLRP3-dependent<br />
protein that inhibits fat breakdown.<br />
While GDF3 is a single upregulated protein<br />
under the overarching control of the NLRP3<br />
inflammasome, NLRP3 itself is required for<br />
fighting off infection, so drugs to block this<br />
protein can increase the risk of certain infections.<br />
Instead, neutralization of GDF3 is an<br />
indirect way to achieve the same goal. If aberrant<br />
inflammation via GDF3 can be prevented,<br />
cells of the adipose tissue will show better<br />
lipolysis. Regardless of the mechanism, these<br />
strategies work to influence the crosstalk<br />
between macrophages and their associated<br />
nerves, which, in turn, influences fat metabolism.<br />
The communication between the nervous,<br />
immune, and metabolic systems that<br />
this research establishes integrates almost<br />
every organ system and so has clear potential<br />
to address the disease of aging as a condition<br />
that impacts all aspects of human health.<br />
When viewed through an even broader<br />
lens, this research is about improving people’s<br />
healthspan, the time during which an individual<br />
is in good health. To clarify the relationship<br />
between catecholamine degradation<br />
and inflammation is to draw the links between<br />
the immune, nervous, and metabolic<br />
systems that, for example, can be implemented<br />
to mitigate neurodegenerative and other<br />
age-related diseases. Ultimately this research<br />
may represent a key to healthier aging. “Our<br />
goal is not to simply increase lifespan,” Dixit<br />
said, “but to add life to the years that exist.”<br />
An understanding of the mechanisms controlling<br />
lipolysis, however narrow of a focus,<br />
can help to achieve this universal ideal: to add<br />
life to your years.<br />
ABOUT THE AUTHOR<br />
CHARLIE MUSOFF<br />
CHARLIE MUSOFF is a sophomore molecular, cellular, and developmental<br />
biology major in Davenport College. Besides being Yale Scientific’s Outreach<br />
Designer, Charlie enjoys running, singing with the Baker’s Dozen, and<br />
teaching with Community Health Educators.<br />
THE AUTHOR WOULD LIKE TO THANK Christina Camell and Professor<br />
Vishwa Deep Dixit for their time and insights.<br />
FURTHER READING<br />
Pirzgalska, Roksana M, et al. “Sympathetic Neuron-Associated Macrophages<br />
Contribute to Obesity by Importing and Metabolizing Norepinephrine.” Nature<br />
Medicine, 9 Oct. 2017, doi:10.1038/nm.4422.<br />
24 Yale Scientific Magazine December 2017 www.yalescientific.org