Comparison of Soil Test Methods, 2009 (pdf) - Palintest
Comparison of Soil Test Methods, 2009 (pdf) - Palintest
Comparison of Soil Test Methods, 2009 (pdf) - Palintest
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Comparison</strong> <strong>of</strong> <strong>Palintest</strong> <strong>Soil</strong> Analysis to External Laboratory Analysis<br />
S. Eddy and S. R. Johnston, <strong>Palintest</strong> Ltd.<br />
This study has been carried out to provide a correlation between the test<br />
methods used in the <strong>Palintest</strong> <strong>Soil</strong> <strong>Test</strong>ing Kits and recognised laboratory<br />
methods for soil analysis.<br />
Where practicable the laboratory method used the same procedure as the<br />
extraction methods recommended in the UK by MAFF/ADAS ‘The Analysis <strong>of</strong><br />
Agricultural Materials’ Reference book 427.<br />
All samples were air dried and passed through a 2mm sieve prior to analysis.<br />
Samples batches were then split prior to testing between methods.<br />
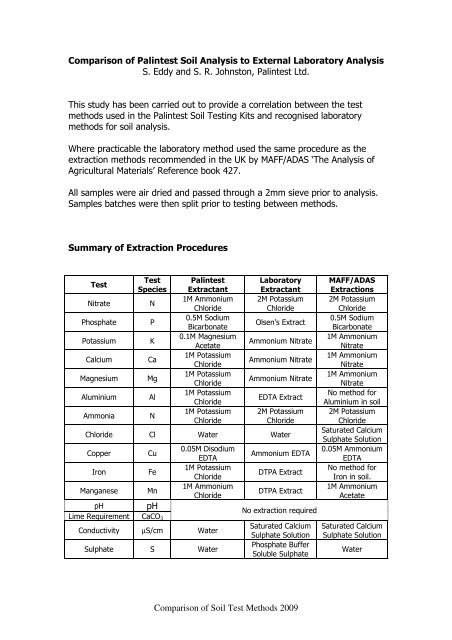
Summary <strong>of</strong> Extraction Procedures<br />
<strong>Test</strong><br />
<strong>Test</strong><br />
Species<br />
Nitrate N<br />
Phosphate P<br />
Potassium K<br />
Calcium Ca<br />
Magnesium Mg<br />
Aluminium Al<br />
Ammonia N<br />
<strong>Palintest</strong><br />
Extractant<br />
1M Ammonium<br />
Chloride<br />
0.5M Sodium<br />
Bicarbonate<br />
0.1M Magnesium<br />
Acetate<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
Laboratory<br />
Extractant<br />
2M Potassium<br />
Chloride<br />
Olsen’s Extract<br />
Ammonium Nitrate<br />
Ammonium Nitrate<br />
Ammonium Nitrate<br />
EDTA Extract<br />
2M Potassium<br />
Chloride<br />
Chloride Cl Water Water<br />
Copper Cu<br />
Iron Fe<br />
Manganese Mn<br />
pH pH<br />
Lime Requirement CaCO3<br />
0.05M Disodium<br />
EDTA<br />
1M Potassium<br />
Chloride<br />
1M Ammonium<br />
Chloride<br />
Conductivity µS/cm Water<br />
Sulphate S Water<br />
Ammonium EDTA<br />
DTPA Extract<br />
DTPA Extract<br />
No extraction required<br />
Saturated Calcium<br />
Sulphate Solution<br />
Phosphate Buffer<br />
Soluble Sulphate<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong><br />
MAFF/ADAS<br />
Extractions<br />
2M Potassium<br />
Chloride<br />
0.5M Sodium<br />
Bicarbonate<br />
1M Ammonium<br />
Nitrate<br />
1M Ammonium<br />
Nitrate<br />
1M Ammonium<br />
Nitrate<br />
No method for<br />
Aluminium in soil<br />
2M Potassium<br />
Chloride<br />
Saturated Calcium<br />
Sulphate Solution<br />
0.05M Ammonium<br />
EDTA<br />
No method for<br />
Iron in soil.<br />
1M Ammonium<br />
Acetate<br />
Saturated Calcium<br />
Sulphate Solution<br />
Water
Results <strong>of</strong> the correlation study<br />
Aluminium<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 2.6 66.2<br />
SWB 1.4 58.6<br />
MSW 1.2 49.7<br />
TKL 1.0 44.5<br />
LRG 1.4 6.8<br />
SLE 2.0 55.5<br />
TAB 1.3 59.6<br />
The <strong>Palintest</strong> aluminium results do not show any linear correlation to the<br />
external laboratory analysis this is due to the extract used to remove the<br />
aluminium from the soil. <strong>Palintest</strong> uses potassium chloride to remove the<br />
available aluminium from the soil and the tablet reagents then measure the<br />
amount <strong>of</strong> free aluminium. The external laboratory extraction method uses<br />
EDTA, which removes the extractable aluminium from the soil, and this is<br />
then determined as complexed aluminium.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Ammonia<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 6.3 1.6<br />
SWB 45.5 47.9<br />
MSW 10.8 12.4<br />
TKL 16.0 16.1<br />
LRG 28.1 25.1<br />
SLE 19.5 19.6<br />
TAB 3.7 0.4<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Calcium<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 2833 2500<br />
SWB 2708 2279<br />
MSW 2792 2339<br />
TKL 1792 1621<br />
LRG 2708 2355<br />
SLE 2208 1751<br />
TAB 2667 1956<br />
The Calcium Tablet Count test has a detection limit <strong>of</strong> ± 250 mg/l.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Chloride<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 67 78<br />
SWB 63 66<br />
MSW 59 48<br />
TKL 59 30<br />
LRG 42 72<br />
SLE 46 36<br />
TAB 59 48<br />
The <strong>Palintest</strong> Chloride test does not show a high level <strong>of</strong> linear correlation to<br />
the external analysis. The reason it does not show a higher level <strong>of</strong> linear<br />
correlation is that the Chloride Tablet Count test has a detection limit <strong>of</strong> ± 25<br />
mg/l. For the soil samples studied in this report the amount <strong>of</strong> chloride<br />
present and the variation between samples was insufficient to demonstrate a<br />
higher degree <strong>of</strong> correlation between the test methods.<br />
Conductivity<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 100 1965<br />
SWB 100 1972<br />
MSW 100 1852<br />
TKL 100 <strong>2009</strong><br />
LRG 100 1959<br />
SLE 100 1920<br />
TAB 100 1999<br />
The <strong>Palintest</strong> Conductivity test does not show a high level <strong>of</strong> linear correlation<br />
to the external analysis due to the extraction procedure used to remove the<br />
soluble salts from the soil. <strong>Palintest</strong> uses deionised water to remove them<br />
and the external method uses a saturated calcium sulphate solution to<br />
displace them from the soil.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Copper<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 5.1 12.5<br />
SWB 54.3 71.7<br />
MSW 7.0 14.1<br />
TKL 7.9 10.1<br />
LRG 8.8 11.6<br />
SLE 15.1 22.5<br />
TAB 5.9 8.7<br />
51023 3.8 2.7<br />
51200 4.1 5.7<br />
52076 8.7 10.2<br />
52078 15.5 18.9<br />
52359 14.0 14.5<br />
53491 23.7 23.1<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Iron<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 41.4 40.4<br />
SWB 44.3 27.4<br />
MSW 51.4 82.2<br />
TKL 50.4 41.9<br />
LRG 19.3 42.4<br />
SLE 37.2 26.6<br />
TAB 51.3 77.6<br />
51025 50.0 92.2<br />
51201 101.6 214.4<br />
51203 112.6 315.3<br />
51930 108.2 404.2<br />
53318 90.5 699.4<br />
The <strong>Palintest</strong> Iron test does not show any linear correlation to the external<br />
analysis due to the extract used to remove the iron from the soil. <strong>Palintest</strong><br />
uses EDTA to remove the available iron from the soil whereas the external<br />
method uses DTPA.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Magnesium<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 151 152<br />
SWB 220 233<br />
MSW 123 112<br />
TKL 240 258<br />
LRG 109 107<br />
SLE 228 221<br />
TAB 302 288<br />
248957 29 20<br />
249344 63 48<br />
249707 101 75<br />
249969 175 150<br />
249254 231 203<br />
249128 232 255<br />
249147 271 301<br />
249496 401 404<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Manganese<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 0.8 2.5<br />
SWB 20.9 19.8<br />
MSW 7.6 3.3<br />
TKL 8.1 4.7<br />
LRG 4.9 2.4<br />
SLE 11.5 5.6<br />
TAB 5.0 4.7<br />
51023 29.9 37.9<br />
51025 17.1 19.3<br />
51202 3.2 6.9<br />
51496 22.4 24.4<br />
51536 54.5 58.7<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Nitrate<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 55.3 58.9<br />
SWB 16.4 26.4<br />
MSW 40.6 36.4<br />
TKL 14.9 14.2<br />
LRG 10.2 7.7<br />
SLE 13.1 11.9<br />
TAB 31.6 35.9<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
pH Block<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 6.3 6.90<br />
SWB 6.8 6.30<br />
MSW 7.6 7.30<br />
TKL 7.8 7.70<br />
LRG 7.8 8.00<br />
SLE 6.8 7.70<br />
TAB 7.6 6.80<br />
249713 4.1 4.06<br />
249890 4.7 4.50<br />
249729 5.6 5.08<br />
248862 5.8 5.51<br />
248778 5.6 6.00<br />
248917 6.8 6.50<br />
248909 7.4 7.00<br />
249044 7.3 7.50<br />
248814 8.0 8.01<br />
The pH block test has a detection limit <strong>of</strong> ± 0.5 pH units.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
pH Meter<br />
Sample<br />
Reference<br />
<strong>Palintest</strong> - pH Probe External Laboratory<br />
249713 4.79 4.06<br />
249890 5.30 4.50<br />
249729 5.69 5.08<br />
248862 5.76 5.51<br />
248778 5.74 6.00<br />
248917 6.64 6.50<br />
248909 7.18 7.00<br />
249044 7.71 7.50<br />
248814 7.96 8.01<br />
SRJ 6.92 6.90<br />
SWB 6.36 6.30<br />
MSW 7.31 7.30<br />
TKL 7.60 7.70<br />
LRG 8.05 8.00<br />
SLE 7.87 7.70<br />
TAB 6.91 6.80<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Phosphate<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 26 41<br />
SWB 29 30<br />
MSW 33 41<br />
TKL 45 48<br />
LRG 77 90<br />
SLE 46 44<br />
TAB 38 40<br />
249017 1 5<br />
249420 13 15<br />
249947 20 20<br />
249540 27 25<br />
249790 28 35<br />
249538 46 40<br />
249652 35 45<br />
248924 41 50<br />
249557 84 80<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Potassium<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 111 92<br />
SWB 218 157<br />
MSW 148 152<br />
TKL 347 377<br />
LRG 141 156<br />
SLE 92 56<br />
TAB 309 330<br />
248730 49 25<br />
248747 77 53<br />
248786 112 102<br />
249190 151 151<br />
249013 212 201<br />
249557 204 245<br />
249547 265 351<br />
249576 309 400<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Sulphate<br />
Sample Reference <strong>Palintest</strong> External Laboratory<br />
SRJ 30.2 20.9<br />
SWB 32.4 32.5<br />
MSW 42.4 17.1<br />
TKL 49.6 22.5<br />
LRG 26.7 29.4<br />
SLE 26.3 33.4<br />
TAB 57.1 30.3<br />
51023 40.0 21.2<br />
51202 180.8 195.5<br />
51318 96.3 56.1<br />
51346 141.0 95.9<br />
The extraction procedure used to remove the water soluble sulphate from the<br />
soil is different for the two methods. <strong>Palintest</strong> uses deionised water and the<br />
external method uses a phosphate buffer.<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong>
Conclusions<br />
Overall the comparison <strong>of</strong> the results between <strong>Palintest</strong> test methods and<br />
external laboratory analysis shows a high degree <strong>of</strong> correlation, with the<br />
exception <strong>of</strong>:<br />
• Aluminium<br />
• Chloride<br />
• Conductivity<br />
• Iron<br />
In the cases <strong>of</strong> aluminium, conductivity and iron the variance is explained by<br />
the difference in extraction method used for these tests.<br />
In the case <strong>of</strong> chloride, no correlation can be concluded due to the small<br />
variance in concentration and degree <strong>of</strong> sensitivity <strong>of</strong> the test method at this<br />
low concentration.<br />
For aluminium and iron there are currently no recognised methods specified<br />
by MAFF/ADAS for the analysis <strong>of</strong> soils.<br />
<strong>Test</strong><br />
<strong>Test</strong><br />
Species<br />
Nitrate N<br />
Phosphate P<br />
Potassium K<br />
Calcium Ca<br />
Magnesium Mg<br />
Aluminium Al<br />
Ammonia N<br />
<strong>Palintest</strong> Method<br />
1M Ammonium<br />
Chloride<br />
0.5M Sodium<br />
Bicarbonate<br />
0.1M Magnesium<br />
Acetate<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
1M Potassium<br />
Chloride<br />
<strong>Comparison</strong> <strong>of</strong> <strong>Soil</strong> <strong>Test</strong> <strong>Methods</strong> <strong>2009</strong><br />
Laboratory<br />
Extractant<br />
Correlation<br />
2M Potassium<br />
Chloride �<br />
Olsen’s Extract �<br />
Ammonium Nitrate �<br />
Ammonium Nitrate �<br />
Ammonium Nitrate �<br />
EDTA Extract<br />
2M Potassium<br />
Chloride<br />
Chloride Cl Water Water<br />
Copper Cu<br />
Iron Fe<br />
Manganese Mn<br />
pH pH<br />
Lime Requirement CaCO3<br />
0.05M Disodium<br />
EDTA<br />
1M Potassium<br />
Chloride<br />
1M Ammonium<br />
Chloride<br />
No extraction<br />
required<br />
Conductivity µS/cm Water<br />
Sulphate S Water<br />
�<br />
Ammonium EDTA �<br />
DTPA Extract<br />
DTPA Extract �<br />
No extraction<br />
required<br />
Saturated Calcium<br />
Sulphate Solution<br />
Phosphate Buffer<br />
Soluble Sulphate<br />
�<br />
�