- Page 2: Organic Synthesis
- Page 8: Copyright © 2007 John Wiley & Sons
- Page 12: vi Contents 28. Kinetic Resolution
- Page 18: Section A: Introduction: Selectivit
- Page 24: 4 1 Planning Organic Syntheses: Tac
- Page 28: 6 1 Planning Organic Syntheses: Tac
- Page 32: 8 1 Planning Organic Syntheses: Tac
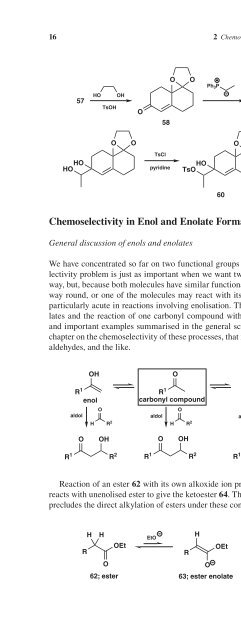
- Page 36: 10 2 Chemoselectivity Chemoselectiv
- Page 40: 12 2 Chemoselectivity Chemoselectiv
- Page 44: 14 2 Chemoselectivity At fi rst sig
- Page 50: Formation of specifi c enol equival
- Page 54: the dilithium derivative of the aci
- Page 58: TiCl 4. The aldehydes 105 and 108,
- Page 62: MeO 2C TsO OH t-BuMe2SiCl OTBDMS CO
- Page 66: formate. The chemoselectivity requi
- Page 72: 28 3 Regioselectivity: Controlled A
- Page 76: 30 3 Regioselectivity: Controlled A
- Page 80: 32 3 Regioselectivity: Controlled A
- Page 84: 34 3 Regioselectivity: Controlled A
- Page 88: 36 3 Regioselectivity: Controlled A
- Page 92: 38 3 Regioselectivity: Controlled A
- Page 96: 40 3 Regioselectivity: Controlled A
- Page 100:
42 3 Regioselectivity: Controlled A
- Page 104:
44 4 Stereoselectivity: Stereoselec
- Page 108:
46 4 Stereoselectivity: Stereoselec
- Page 112:
48 4 Stereoselectivity: Stereoselec
- Page 116:
50 4 Stereoselectivity: Stereoselec
- Page 120:
52 4 Stereoselectivity: Stereoselec
- Page 124:
54 4 Stereoselectivity: Stereoselec
- Page 128:
56 5 Alternative Strategies for Eno
- Page 132:
58 5 Alternative Strategies for Eno
- Page 136:
60 5 Alternative Strategies for Eno
- Page 140:
62 5 Alternative Strategies for Eno
- Page 144:
64 5 Alternative Strategies for Eno
- Page 148:
66 5 Alternative Strategies for Eno
- Page 152:
68 5 Alternative Strategies for Eno
- Page 158:
6 Choosing a Strategy: The Synthesi
- Page 162:
this a good strategy, particularly
- Page 166:
Modern versions of these strategies
- Page 170:
The Nazarov Reaction In addition to
- Page 174:
If iron carbonyl is used to generat
- Page 178:
Intramolecular Pauson-Khand reactio
- Page 182:
Other recent developments include a
- Page 186:
medicinal chemists 43 to make analo
- Page 190:
6 References 87 15. P. E. Eaton and
- Page 198:
7 The Ortho Strategy for Aromatic C
- Page 202:
Derivatives of meta cresol 10 are e
- Page 206:
ond under the reaction conditions s
- Page 210:
Firstly, let us consider the substi
- Page 214:
would simply undergo a lithium - ha
- Page 218:
Et2N O O In the same way, direct co
- Page 222:
eacted with BuLi followed by solid
- Page 226:
115 ii) Dilithiations OCONEt 2 1. s
- Page 230:
The aldehyde 129 is attacked using
- Page 234:
Benzyne Formation—A different aro
- Page 238:
The lactone 168 is lithiated at the
- Page 242:
8 σ-Complexes of Metals Introducti
- Page 246:
R Br 5 8 Introduction The structure
- Page 250:
Chiral Grignard reagents It has lon
- Page 254:
43 Me R FGA Transition Metal Comple
- Page 258:
complex with the two π-electrons o
- Page 262:
more stable than Mg(0), as in the m
- Page 266:
Just occasionally a Pd reaction wit
- Page 270:
9 Controlling the Michael Reaction
- Page 274:
Where direct (1,2-) addition is nec
- Page 278:
9 Using Copper (I) to Achieve Micha
- Page 282:
O OLi Me2CuLi 57 58 PhCHO 59; 1:2 s
- Page 286:
81; β-panasinene Wittig 2 + 2 O O
- Page 290:
Do not suppose that the regioselect
- Page 294:
10 Specifi c Enol Equivalents Intro
- Page 298:
O 16 CO2t-Bu 15 cat t-BuOK t-BuOH O
- Page 302:
Alkylation with simple alkyl halide
- Page 306:
HO + OMe HO OMe This time the lithi
- Page 310:
Aldehydes 88 do not form stable lit
- Page 314:
The second 119 clearly came from th
- Page 318:
now that the simple enols and enola
- Page 322:
of the ketone 152 with an aldehyde
- Page 328:
156 11 Extended Enolates R O O O O
- Page 332:
158 11 Extended Enolates There is n
- Page 336:
160 11 Extended Enolates The PhS gr
- Page 340:
162 11 Extended Enolates acrylate g
- Page 344:
164 11 Extended Enolates cleanly in
- Page 348:
166 11 Extended Enolates The Baylis
- Page 352:
168 11 Extended Enolates A Baylis-H
- Page 356:
170 11 Extended Enolates Conclusion
- Page 362:
12 Allyl Anions Introduction: Allyl
- Page 366:
If neither the halides nor their me
- Page 370:
Similar compounds 56 can be made 13
- Page 374:
see 21 and 23, from which a mixture
- Page 378:
Me 3Si Et 2O.BF 3 There are many re
- Page 382:
OH Co(dmgH) 2py SiMe3 (S)-138 0.3 e
- Page 386:
R 1 The best candidates are phospho
- Page 390:
12 References 187 13. H. Mattes and
- Page 396:
190 13 Homoenolates B B Direct homo
- Page 400:
192 13 Homoenolates MeO OSiMe 3 Con
- Page 404:
194 13 Homoenolates MeO I 57 NHBoc
- Page 408:
196 13 Homoenolates Sulfones Possib
- Page 412:
198 13 Homoenolates react α (or wh
- Page 416:
200 13 Homoenolates N Li An open ch
- Page 420:
202 13 Homoenolates 5. Disconnectio
- Page 424:
204 14 Acyl Anion Equivalents O R C
- Page 428:
206 14 Acyl Anion Equivalents An op
- Page 432:
208 14 Acyl Anion Equivalents Dithi
- Page 436:
210 14 Acyl Anion Equivalents EEO C
- Page 440:
212 14 Acyl Anion Equivalents Lithi
- Page 444:
214 14 Acyl Anion Equivalents Synth
- Page 448:
216 14 Acyl Anion Equivalents A syn
- Page 452:
218 14 Acyl Anion Equivalents Catal
- Page 456:
220 14 Acyl Anion Equivalents 30. J
- Page 462:
15 Synthesis of Double Bonds of Def
- Page 466:
15 Control of Alkene Geometry by Eq
- Page 470:
Each of the following reactions giv
- Page 474:
at right angles to each other. Prot
- Page 478:
RCHO Br Ph3P MeCN PPh3 BuLi DMSO 71
- Page 482:
O (EtO) 2P O OEt EtO or NaH O (EtO)
- Page 486:
A more serious example is from John
- Page 490:
Ph3P R1 NaOH Ph2P R1 O BuLi Ph2P R1
- Page 494:
R2 R1 PhSiMe2 Me OH 142 KH 18-crown
- Page 498:
The Kocienski modifi cation of the
- Page 502:
MeO Ph O O TiCl3, Li MeO O naphthal
- Page 506:
A much simpler heterocyclic eight-m
- Page 510:
[2,3]-Sigmatropic rearrangements: T
- Page 514:
Stereospecifi c Methods for Z-Alken
- Page 518:
A synthesis of methoxatin Methoxati
- Page 522:
References 15 References 253 Genera
- Page 526:
16 Stereo-Controlled Vinyl Anion Eq
- Page 530:
Both the Grignard reagent 14 and vi
- Page 534:
The vinyl-lithium may be combined d
- Page 538:
hν Me H 2N NHTs CH2 CH2 MeOH 2. DM
- Page 542:
This example of the commoner thermo
- Page 546:
Hydroalumination of propargyl alcoh
- Page 550:
Here is an example from the McMurry
- Page 554:
Reactions of Vinyl Sn, B, Al, Si, a
- Page 558:
O CHO 173 Reactivity of vinyl alane
- Page 562:
Me 3Si E electrophilic attack O SiM
- Page 566:
References 16 References 275 Genera
- Page 570:
17 Electrophilic Attack on Alkenes
- Page 574:
Chemoselectivity The commonest form
- Page 578:
HO Controlling Chemoselectivity 17
- Page 582:
‘Markovnikov’ Hydration Mercura
- Page 586:
The stereochemistry of all these st
- Page 590:
There is good stereoselectivity wit
- Page 594:
Me 3SiCl gives a stable silyl enol
- Page 598:
MeO2C Br H2O H Br H 150 O MeO O O O
- Page 602:
stereogenic centres around a six-me
- Page 606:
determined by the proximity of the
- Page 610:
OH O CO2Et from ethyl acetoacetate
- Page 614:
periodic table) and that gives the
- Page 618:
225 second CO migrationR R first B
- Page 622:
y the proximity of the right alkene
- Page 626:
17 References 305 5. A. Armstrong a
- Page 632:
308 18 Vinyl Cations: Palladium-Cat
- Page 636:
310 18 Vinyl Cations: Palladium-Cat
- Page 640:
312 18 Vinyl Cations: Palladium-Cat
- Page 644:
314 18 Vinyl Cations: Palladium-Cat
- Page 648:
316 18 Vinyl Cations: Palladium-Cat
- Page 652:
318 18 Vinyl Cations: Palladium-Cat
- Page 656:
320 18 Vinyl Cations: Palladium-Cat
- Page 660:
322 18 Vinyl Cations: Palladium-Cat
- Page 664:
324 18 Vinyl Cations: Palladium-Cat
- Page 668:
326 18 Vinyl Cations: Palladium-Cat
- Page 672:
328 18 Vinyl Cations: Palladium-Cat
- Page 676:
330 18 Vinyl Cations: Palladium-Cat
- Page 680:
332 18 Vinyl Cations: Palladium-Cat
- Page 684:
334 18 Vinyl Cations: Palladium-Cat
- Page 688:
336 18 Vinyl Cations: Palladium-Cat
- Page 694:
19 Allyl Alcohols: Allyl Cation Equ
- Page 698:
This can be useful when oxidation o
- Page 702:
in chapters 2-4 and summarised in c
- Page 706:
[2,3] Sigmatropic shifts in the all
- Page 710:
The [2,3] Wittig rearrangement Ethe
- Page 714:
Ph Ph CH 2I 2, Zn Ph Ph cat Cu cat
- Page 718:
By contrast, the diene 132 with one
- Page 722:
6 R 5 4 CO2Me 3 [3,3] CO 2Me 1 2 OS
- Page 726:
3 O 1 O 2 O OR O O OR O O OR O 4 5
- Page 730:
the ring) with the bulk of the side
- Page 734:
The scope of the Claisen-Cope rearr
- Page 738:
In three dimensions the reaction is
- Page 742:
Conjugating and electron-withdrawin
- Page 746:
Now for the stereochemistry. If a s
- Page 750:
19 References 367 4. B. M. Trost an
- Page 758:
20 Control of Stereochemistry - Int
- Page 762:
1 achiral If we just imagine the si
- Page 766:
1 achiral 5 achiral How to decide i
- Page 770:
What we draw What it means OH OH OH
- Page 774:
Achiral diastereomers You do not ne
- Page 778:
not convinced by this, make a tetra
- Page 782:
image at all. Since organic chemist
- Page 786:
e turned into an asymmetric synthes
- Page 790:
Step No. 3 Decide whether or not th
- Page 794:
addition of a nucleophile to ethyl
- Page 798:
this, your de could be 0% (which wo
- Page 802:
Since two asymmetric reactions are
- Page 806:
that chirotopicity (something that
- Page 810:
D D Cp 2Zr(H)Cl 93 D Ph 20 Popular
- Page 814:
21 Controlling Relative Stereochemi
- Page 818:
21 Formation of Cyclic Compounds vi
- Page 822:
18 Curtius CO 2H rearrangement 1. C
- Page 826:
Nomenclature Just before we go any
- Page 830:
Getting control - ketones It is all
- Page 834:
Getting control - esters With ester
- Page 838:
axial or equatorial leading to the
- Page 842:
21 Reactions of Cyclic Compounds: C
- Page 846:
Nu atoms move towards each other Nu
- Page 850:
will provide a barrier to incoming
- Page 854:
obvious cousin must be the bromolac
- Page 858:
i-Pr OH i-Pr (±)-139 Me resolution
- Page 862:
But fi rst consider the yield of th
- Page 866:
other. With a kinetic diastereosele
- Page 870:
molecule 184. The butyl group does
- Page 874:
21 Open Chain Chemistry - in its Mo
- Page 878:
Cl 211a 21 Open Chain Chemistry - i
- Page 882:
Reaction with electrophiles can be
- Page 886:
22 Resolution Resolution Introducti
- Page 890:
The amine 2 is made by a chemical r
- Page 894:
One of the functional groups now sh
- Page 898:
The resolving agent must now be rem
- Page 902:
Resolution of Diastereoisomers When
- Page 906:
Resolution with half an equivalent
- Page 910:
malaria, but that it also had fewer
- Page 914:
Racemic Compounds Each crystal cont
- Page 918:
Finding a differential crystallisat
- Page 922:
When the N-acetyl derivative of the
- Page 926:
oom temperature by equilibration wi
- Page 930:
The only chiral intermediate is the
- Page 934:
(±)-129 OH OAc Pseudomonas lipase
- Page 938:
At 50% conversion, the product cont
- Page 942:
H O It should not be assumed that a
- Page 946:
23 The Chiral Pool — Asymmetric S
- Page 950:
R O OH Ph If you are manufacturing
- Page 954:
Valinol 27 and phenylalaninol 29 ar
- Page 958:
Derivatives of these aminoalcohols
- Page 962:
Formaldehyde introduces one carbon
- Page 966:
OH OBn OTBDMS TBDMSO OBn OH 108; pr
- Page 970:
The synthesis of captopril The fi r
- Page 974:
Normal peptide coupling (N-Boc-Ala
- Page 978:
The synthesis of ()-laurencin Some
- Page 982:
The next few steps including the vi
- Page 986:
That leaves just one nucleophile to
- Page 990:
231 O 2N The New Chiral Pool Glycid
- Page 994:
O HN Ph NH O When they wanted a sin
- Page 998:
Ph O H H O Me H O N N Me Ph (EtO) P
- Page 1002:
The synthesis of crixivan (Indinavi
- Page 1006:
O While 319 could be converted into
- Page 1010:
Now the terminal OH group can be de
- Page 1014:
Derived from them and from other ch
- Page 1018:
HO The only C 3 sugar is the rather
- Page 1022:
23 References 503 J. Capiello, Tetr
- Page 1028:
506 24 Asymmetric Induction I Reage
- Page 1032:
508 24 Asymmetric Induction I Reage
- Page 1036:
510 24 Asymmetric Induction I Reage
- Page 1040:
512 24 Asymmetric Induction I Reage
- Page 1044:
514 24 Asymmetric Induction I Reage
- Page 1048:
516 24 Asymmetric Induction I Reage
- Page 1052:
518 24 Asymmetric Induction I Reage
- Page 1056:
520 24 Asymmetric Induction I Reage
- Page 1060:
522 24 Asymmetric Induction I Reage
- Page 1064:
524 24 Asymmetric Induction I Reage
- Page 1070:
25 Asymmetric Induction II Asymmetr
- Page 1074:
hydroperoxides can be used), a cata
- Page 1078:
O 10a Mesylate was chosen as the le
- Page 1082:
carbon, that is C-1 in 25. The mino
- Page 1086:
H H H NHCHO O Cl 45; kalihinene X H
- Page 1090:
64 py HCl HCl Ar OH CO2Me Summary o
- Page 1094:
Osmium tetroxide is very expensive
- Page 1098:
R R step A R O O OH O R O O Os O O
- Page 1102:
Recommended ligands for different c
- Page 1106:
ecause the secondary alcohols are t
- Page 1110:
Note that to keep a straight carbon
- Page 1114:
25 Dihydroxylating Compounds with M
- Page 1118:
25 Dihydroxylating Compounds with M
- Page 1122:
The reaction in the box below shows
- Page 1126:
The synthesis of diltiazem 42 start
- Page 1130:
MeCN H 2SO 4 SO 3 3 mol% 203 N O Me
- Page 1134:
We have seen much of quinine and qu
- Page 1138:
Consider, for example, a hypothetic
- Page 1142:
Me OH 257 2. 1. SnCl 4, (CH 2O) n 2
- Page 1146:
25 References 565 10. H. Miyaoka, H
- Page 1150:
26 Asymmetric Induction III Asymmet
- Page 1154:
Horner 4 at Mainz in Germany and Kn
- Page 1158:
26 Hydrogenation with C 2-Symmetric
- Page 1162:
When asymmetric reduction of the ke
- Page 1166:
Corey’s CBS Reduction of Ketones
- Page 1170:
Nowadays much less catalyst is need
- Page 1174:
This was an extraordinary result wh
- Page 1178:
CO2H O Me N Ph CHO Bn Bn NH2 phenyl
- Page 1182:
Ph CHO More recent developments inc
- Page 1186:
Cyclopropanation Historically one o
- Page 1190:
N Ph Ph *Mo R 154 155 156 Mo* Examp
- Page 1194:
Synthesis of enalapril The ACE (Ang
- Page 1198:
Recent improvements include the rep
- Page 1202:
O NR 2 N CHO i-Pr 2Zn O NR 2 Non-li
- Page 1206:
Enantioselectivity with open chain
- Page 1210:
26 References 597 22. T. D. Machaje
- Page 1216:
600 27 Asymmetric Induction IV Subs
- Page 1220:
602 27 Asymmetric Induction IV Subs
- Page 1224:
604 27 Asymmetric Induction IV Subs
- Page 1228:
606 27 Asymmetric Induction IV Subs
- Page 1232:
608 27 Asymmetric Induction IV Subs
- Page 1236:
610 27 Asymmetric Induction IV Subs
- Page 1240:
612 27 Asymmetric Induction IV Subs
- Page 1244:
614 27 Asymmetric Induction IV Subs
- Page 1248:
616 27 Asymmetric Induction IV Subs
- Page 1252:
618 27 Asymmetric Induction IV Subs
- Page 1256:
620 27 Asymmetric Induction IV Subs
- Page 1260:
622 27 Asymmetric Induction IV Subs
- Page 1264:
624 27 Asymmetric Induction IV Subs
- Page 1270:
28 Kinetic Resolution Types of Reac
- Page 1274:
Starting Materials A selective whee
- Page 1278:
Other symbols used in the realm of
- Page 1282:
11 N R R OH 12 99% ee at 55% conver
- Page 1286:
With the fast-reacting enantiomer t
- Page 1290:
Dynamic Kinetic Resolutions The big
- Page 1294:
MeO 2C S OAc (±)-40 OEt OEt 28 Dyn
- Page 1298:
OH O 51 Hydrogenation Something tha
- Page 1302:
9 OH Lipase 28 Parallel Kinetic Res
- Page 1306:
N N Li Ph Me (R)-76 base 77 : 78 LD
- Page 1310:
TfO OTf 82 N Ph Cl Pd Cl N 85 28 Do
- Page 1314:
89 Triene 89 reacts to form the dio
- Page 1318:
29 Enzymes: Biological Methods in A
- Page 1322:
O 1; 40 g CO 2Et 200 g baker's yeas
- Page 1326:
In some cases two different enzymes
- Page 1330:
difference. One useful group is the
- Page 1334:
This is a kinetic resolution that w
- Page 1338:
the 2,3-bond in the ring. Bromobenz
- Page 1342:
Br O O Epoxidation There are all so
- Page 1346:
Whole cells of engineered E. coli B
- Page 1350:
Enzymatically catalysed aldol react
- Page 1354:
160 [O] Engineered aldolase O O O H
- Page 1358:
RO 181 1. 9-BBN 2. 175; R = Ac [PdC
- Page 1362:
The synthetic compounds are obvious
- Page 1366:
Racemic 217 could readily be acetyl
- Page 1370:
now Cbz derivatised, remains in the
- Page 1374:
29 References 679 42. B. B. Corson,
- Page 1380:
682 30 New Chiral Centres from Old
- Page 1384:
684 30 New Chiral Centres from Old
- Page 1388:
686 30 New Chiral Centres from Old
- Page 1392:
688 30 New Chiral Centres from Old
- Page 1396:
690 30 New Chiral Centres from Old
- Page 1400:
692 30 New Chiral Centres from Old
- Page 1404:
694 30 New Chiral Centres from Old
- Page 1408:
696 30 New Chiral Centres from Old
- Page 1412:
698 30 New Chiral Centres from Old
- Page 1416:
700 30 New Chiral Centres from Old
- Page 1420:
702 30 New Chiral Centres from Old
- Page 1424:
704 30 New Chiral Centres from Old
- Page 1428:
706 30 New Chiral Centres from Old
- Page 1432:
708 30 New Chiral Centres from Old
- Page 1436:
710 30 New Chiral Centres from Old
- Page 1440:
712 30 New Chiral Centres from Old
- Page 1444:
714 30 New Chiral Centres from Old
- Page 1450:
31 Strategy of Asymmetric Synthesis
- Page 1454:
Ph The racemic amino alcohol 2 was
- Page 1458:
a Pd-catalysed coupling of a vinyl
- Page 1462:
PART III - GRANDISOL AND SOME BICYC
- Page 1466:
The remainder of the synthesis invo
- Page 1470:
These drugs are clearly peptide mim
- Page 1474:
PART V - CONFORMATIONAL CONTROL AND
- Page 1478:
The epoxide 95 was reduced with RED
- Page 1482:
100 O O An alternative approach 17
- Page 1486:
It works by the spontaneous hydroly
- Page 1490:
The aldol reaction proved diffi cul
- Page 1494:
A cunning solution was to attach th
- Page 1498:
In previous syntheses this last cen
- Page 1502:
MeO2C 186 1 mol% K2OsO2(OH)4 1 mol%
- Page 1506:
References 31 References 745 Genera
- Page 1514:
32 Functionalisation of Pyridine Th
- Page 1518:
So far electrophilic attack on pyri
- Page 1522:
F F 32 Regioselectivity in Electrop
- Page 1526:
The Anti-Tumour Antibiotic Kedarcid
- Page 1530:
y chloride 67 occurs, it is the mos
- Page 1534:
Omeprazole: Synthesis 1 The diffi c
- Page 1538:
N 107 Diazines CO 2H 1. LiTMP 2. CO
- Page 1542:
Tandem Double Lithiation: The asymm
- Page 1546:
PART III - SURPRISINGLY SUCCESSFUL
- Page 1550:
NMR of compound 171 can be seen. Yo
- Page 1554:
N OH CO2H OO 2S Extension by Vicari
- Page 1558:
N 204 NH 2 NH 2 H 2 catalyst N H N
- Page 1562:
Boc N 239 O Alternatively, coupling
- Page 1566:
32 References 775 35. F. I. Carroll
- Page 1572:
778 33 Oxidation of Aromatic Compou
- Page 1576:
780 33 Oxidation of Aromatic Compou
- Page 1580:
782 33 Oxidation of Aromatic Compou
- Page 1584:
784 33 Oxidation of Aromatic Compou
- Page 1588:
786 33 Oxidation of Aromatic Compou
- Page 1592:
788 33 Oxidation of Aromatic Compou
- Page 1596:
790 33 Oxidation of Aromatic Compou
- Page 1600:
792 33 Oxidation of Aromatic Compou
- Page 1604:
794 33 Oxidation of Aromatic Compou
- Page 1608:
796 33 Oxidation of Aromatic Compou
- Page 1612:
798 33 Oxidation of Aromatic Compou
- Page 1616:
800 33 Oxidation of Aromatic Compou
- Page 1620:
802 33 Oxidation of Aromatic Compou
- Page 1624:
804 33 Oxidation of Aromatic Compou
- Page 1628:
806 33 Oxidation of Aromatic Compou
- Page 1632:
808 33 Oxidation of Aromatic Compou
- Page 1636:
810 34 Functionality and Pericyclic
- Page 1640:
812 34 Functionality and Pericyclic
- Page 1644:
814 34 Functionality and Pericyclic
- Page 1648:
816 34 Functionality and Pericyclic
- Page 1652:
818 34 Functionality and Pericyclic
- Page 1656:
820 34 Functionality and Pericyclic
- Page 1660:
822 34 Functionality and Pericyclic
- Page 1664:
824 34 Functionality and Pericyclic
- Page 1668:
826 34 Functionality and Pericyclic
- Page 1672:
828 34 Functionality and Pericyclic
- Page 1676:
830 34 Functionality and Pericyclic
- Page 1680:
832 34 Functionality and Pericyclic
- Page 1686:
35 Synthesis and Chemistry of Azole
- Page 1690:
we shall be looking at the synthesi
- Page 1694:
The synthesis of allopurinol Many h
- Page 1698:
. Routes to Isoxazoles The synthesi
- Page 1702:
Better results are often obtained b
- Page 1706:
Zolterine (anti-hypertensive) Analy
- Page 1710:
tetrazole 122 is about as strongly
- Page 1714:
unsymmetrical anion, reasonable reg
- Page 1718:
You may be surprised by the stereos
- Page 1722:
N N X X N X N X X N X pyridazine (X
- Page 1726:
in a typical Heck reaction without
- Page 1730:
X N O N H CHO + 204a EtO2C N 211 S
- Page 1734:
SnCl2 HCl Now the seven-membered ri
- Page 1738:
35 References 861 8. R. N. Butler i
- Page 1744:
864 36 Tandem Organic Reactions Asy
- Page 1748:
866 36 Tandem Organic Reactions by
- Page 1752:
868 36 Tandem Organic Reactions Reg
- Page 1756:
870 36 Tandem Organic Reactions Ste
- Page 1760:
872 36 Tandem Organic Reactions Tun
- Page 1764:
874 36 Tandem Organic Reactions O E
- Page 1768:
876 36 Tandem Organic Reactions O H
- Page 1772:
878 36 Tandem Organic Reactions The
- Page 1776:
880 36 Tandem Organic Reactions Tan
- Page 1780:
882 36 Tandem Organic Reactions Tan
- Page 1784:
884 36 Tandem Organic Reactions Tan
- Page 1788:
886 36 Tandem Organic Reactions O O
- Page 1792:
888 36 Tandem Organic Reactions 209
- Page 1796:
890 36 Tandem Organic Reactions PAR
- Page 1800:
892 36 Tandem Organic Reactions R 1
- Page 1806:
Index Acetal, 195 Selective formati
- Page 1810:
Bombykol, 232 Boranes, 256, 264, 29
- Page 1814:
with azadienes, 811-814 with imines
- Page 1818:
Erythrina alkaloid, 134, 876 Esomep
- Page 1822:
Kalihinene X ()-α-Kainic acid, 357
- Page 1826:
Nootkatone, 50 Norvir, 479 NOVRAD,
- Page 1830:
Seebach, 55, 56, 600 Relay chiral u
- Page 1834:
Vinyl alanes, 271 Vinyl anion equiv