3. AIM OF THE SURVEILLANCE
3. AIM OF THE SURVEILLANCE
3. AIM OF THE SURVEILLANCE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
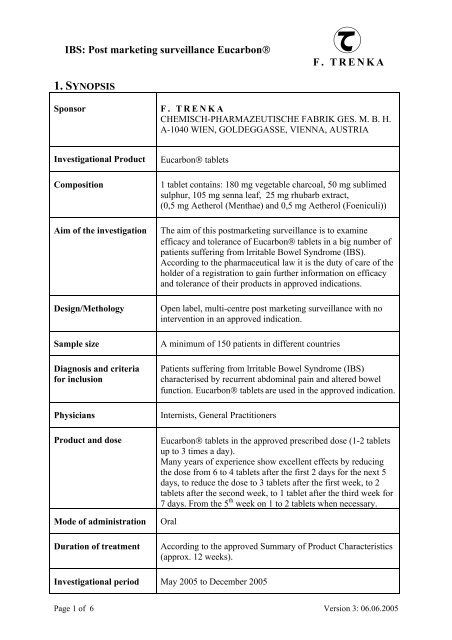
IBS: Post marketing surveillance Eucarbon®<br />
1. SYNOPSIS<br />
Sponsor<br />
Investigational Product<br />
Composition<br />
Aim of the investigation<br />
Design/Methology<br />
Sample size<br />
Diagnosis and criteria<br />
for inclusion<br />
Physicians<br />
Product and dose<br />
Mode of administration<br />
Duration of treatment<br />
Investigational period<br />
F. TRENKA<br />
F. TRENKA<br />
CHEMISCH-PHARMAZEUTISCHE FABRIK GES. M. B. H.<br />
A-1040 WIEN, GOLDEGGASSE, VIENNA, AUSTRIA<br />
Eucarbon® tablets<br />
1 tablet contains: 180 mg vegetable charcoal, 50 mg sublimed<br />
sulphur, 105 mg senna leaf, 25 mg rhubarb extract,<br />
(0,5 mg Aetherol (Menthae) and 0,5 mg Aetherol (Foeniculi))<br />
The aim of this postmarketing surveillance is to examine<br />
efficacy and tolerance of Eucarbon® tablets in a big number of<br />
patients suffering from lrritable Bowel Syndrome (IBS).<br />
According to the pharmaceutical law it is the duty of care of the<br />
holder of a registration to gain further information on efficacy<br />
and tolerance of their products in approved indications.<br />
Open label, multi-centre post marketing surveillance with no<br />
intervention in an approved indication.<br />
A minimum of 150 patients in different countries<br />
Patients suffering from lrritable Bowel Syndrome (IBS)<br />
characterised by recurrent abdominal pain and altered bowel<br />
function. Eucarbon® tablets are used in the approved indication.<br />
Internists, General Practitioners<br />
Eucarbon® tablets in the approved prescribed dose (1-2 tablets<br />
up to 3 times a day).<br />
Many years of experience show excellent effects by reducing<br />
the dose from 6 to 4 tablets after the first 2 days for the next 5<br />
days, to reduce the dose to 3 tablets after the first week, to 2<br />
tablets after the second week, to 1 tablet after the third week for<br />
7 days. From the 5 th week on 1 to 2 tablets when necessary.<br />
Oral<br />
According to the approved Summary of Product Characteristics<br />
(approx. 12 weeks).<br />
May 2005 to December 2005<br />
Page 1 of 6 Version 3: 06.06.2005
IBS: Post marketing surveillance Eucarbon®<br />
2. INTRODUCTION<br />
F. TRENKA<br />
Irritable bowel syndrome (IBS) is a benign relapsing chronic disorder, characterised by<br />
recurrent abdominal pain and altered bowel function. It is estimated that 9 to 22% of the<br />
general population has clinical symptoms of IBS but only about 5% seek medical care.<br />
IBS is the most common diagnosis made by gastroenterologists and accounts for<br />
approximately 50% of all referrals. IBS contributes significantly to disability, days off work<br />
or school and health care costs.<br />
IBS is considered as a complex disease whereby clinical and therapeutical management is<br />
particularly difficult. There is however no evidence that IBS predisposes to cancer or any<br />
other disorder. Patients complain of general symptoms of abdominal pain (most frequently<br />
located in the lower left quadrant), changes in bowel habits/ stools, (e.g., stools may be softformed<br />
with pencil-size diameter), flatulence and / or abdominal distension with the onset of<br />
symptoms usually weeks or months prior to seeking medical attention.<br />
The management of irritable bowel syndrome is quite complex. Bile acid-binding agents such<br />
as cholestyramine have been successfully used. The beneficial effect of this treatment may be<br />
a result of the general constipation effect of the resin. Furthermore it has been proposed that<br />
elevated levels of short-chain fatty acids in the stools may mediate the diarrhea in irritable<br />
bowel syndrome, however symptoms have not correlated with fecal levels.<br />
The active ingredients contained in Eucarbon® are exclusively of vegetable and mineral origin. The<br />
effect of Eucarbon® is twofold: it regulates the bowel functions and acts as a mild laxative. The<br />
vegetable charcoal adsorbs toxic substances originating from bacteria and from metabolic processes.<br />
Eucarbon® is a combination of senna, rhubarb, carbo ligni and sulfur. It belongs by its<br />
pharmacological and pharmacodynamical properties to the stimulant laxatives, but due to the mild<br />
adsorptive activity of carbo ligni Eucarbon® also is a mild adsorbent and therefore a medicine<br />
against general digestive disorders.<br />
Laxatives such as Eucarbon® may increase intestinal motility via decreased absorption of salt<br />
and water secondary to a decrease in transit time.<br />
Eucarbon® was developed in 1909 by the pharmacist Mag. F. Trenka and by Prof. Dr. W.<br />
Pauli. Eucarbon® tablets contain only vegetable and mineral active ingredients and are<br />
produced with up-to-date production methods in accordance with GMP-Standards.<br />
Eucarbon® is on the market for several years in different European and non-European<br />
countries, such as Morocco, South Africa and Indonesia.<br />
Page 2 of 6 Version 3: 06.06.2005
IBS: Post marketing surveillance Eucarbon®<br />
<strong>3.</strong> <strong>AIM</strong> <strong>OF</strong> <strong>THE</strong> <strong>SURVEILLANCE</strong><br />
F. TRENKA<br />
The aim of this postmarketing surveillance is to examine efficacy and tolerability of<br />
Eucarbon ® tablets in patients suffering from Irritable Bowel Syndrome (IBS).<br />
Due to the character of a post marketing surveillance no defined investigations are proposed.<br />
Eucarbon® is used according to the approved guidelines given in the SPC (summary of<br />
product characteristics). There is no therapeutic measure proposed, which goes beyond the<br />
usual routine of medical care.<br />
4. TREATMENT AND MEDICATION<br />
Eucarbon® is used according to the approved indication and dosage given in the SPC.<br />
Composition:<br />
1 tablet contains:<br />
Carbo Ligni – Wood Charcoal 180,00mg<br />
Sulfur depuratum – Sublimed Sulfur 50,00 mg<br />
Fol. sennae – Senna Leaf 105,00 mg<br />
Extractum Rhei – Rhubarb Extract 25,00 mg<br />
(Aetherol Menthae 0,5 mg)<br />
(Aetherol Foeniculi 0,5 mg)<br />
Prescription: over the counter (OTC)<br />
Presentation (Packaging): 30, 50, 100 and 500 tablets<br />
Shelf life: 60 months<br />
Dose: 1 to 2 tablets up to 3 times a day<br />
Many years of experience show excellent effects by reducing the initial dose from 6 to 4<br />
tablets after the first 2 days for the next 5 days. The reduction of 1 tablet per day should<br />
maintain from the second week on. After the 4 th week, from the 5 th week on 1 to 2 tablets are<br />
prescribed when necessary.<br />
5. DESIGN <strong>OF</strong> <strong>THE</strong> <strong>SURVEILLANCE</strong><br />
The total duration of the PMS will be 7 months. The investigation starts in May 2005 and will<br />
end in December 2005.<br />
Patients will be treated with Eucarbon® according to the approved SPC for approximately 12<br />
weeks. Approximately 6 weeks after the baseline visit the first control visit will be performed.<br />
After additional 6 weeks (a total of 12 weeks) a second control visit will be conducted.<br />
Page 3 of 6 Version 3: 06.06.2005
IBS: Post marketing surveillance Eucarbon®<br />
F. TRENKA<br />
The results of each visit of the patient will be reported in the provided case report forms<br />
(CRFs).<br />
6. SUBJECTS<br />
The investigation will be conducted with patients with Irritable Bowel Syndrome (IBS).<br />
Patients with well known hypersensitivities or allergic reactions against components of<br />
Eucarbon® tablets must be excluded from the post marketing surveillance. There are no<br />
restrictions to the physicians choice of therapy.<br />
At any time the investigator can withdraw by his discretion the patient from the surveillance.<br />
7. EFFICACY AND TOLERANCE<br />
7.1. Efficacy<br />
The main parameter for evaluating the efficacy of Eucarbon® is the change in symptoms<br />
from baseline to the end of the PMS.<br />
Furthermore the change in the severity of the illness and an overall assessment of the<br />
effectiveness will be evaluated.<br />
7.2. Tolerance<br />
Parameters for evaluating the tolerance of Eucarbon® tablets are clinical findings and a<br />
global evaluation of tolerance by the physician and the patient. All AEs will be listed and all<br />
reasons for discontinuation of the treatment will be documented.<br />
If an Adverse Event (AE) occurs, the AE Reporting Page must be completed. All new<br />
indispositions occurring between baseline visit and end of visit (intercurrent illnesses) are<br />
handled as adverse events and have to be reported on the adverse event reporting page.<br />
A serious adverse event (SAE) is defined as an adverse event, which is observed during any<br />
phase of the intake of Eucarbon®, independently of its dosage and fulfils one of the following<br />
criteria:<br />
• results in death<br />
• is immediately life-threatening<br />
• requires or prolongs patient hospitalisation<br />
• results in permanent diability/incapacity of the patient<br />
• development of a new tumour<br />
• congenital anomaly/birth defect<br />
Page 4 of 6 Version 3: 06.06.2005
IBS: Post marketing surveillance Eucarbon®<br />
F. TRENKA<br />
Reporting of SUSARS<br />
Suspected Unexpected Serious Adverse Reactions are SAEs where a causal relationship with<br />
the administration of Eucarbon® cannot be excluded. SUSARs have to be reported within one<br />
working day per telephone or fax to the responsible person of TRENKA GmbH:<br />
To: Herr Dr. E.H Moser<br />
Fa. TRENKA GmbH<br />
Goldeggasse 5<br />
A-1040 Wien<br />
Telephone: +43-1-505 30 53-0<br />
Fax: +43-1-505 30 53-31<br />
E – mail: office@eucarbon.at<br />
8. INFORMATION AND PATIENTS INFORMED CONSENT<br />
Because all patients are treated within the limitations of the SPC information of the patient<br />
going beyond the medical obligation of the physician is not necessary during the PMS.<br />
9. ETHICAL ASPECTS<br />
For the conduction of this post marketing surveillance no approval of the local Ethic<br />
committees or other authorities are needed.<br />
To maintain confidentiality of the patient data the name/data of the patients will only be<br />
transmitted anonymized by their physician. The coding is done in a way, which makes a<br />
reconstruction of the patients name impossible.<br />
10. MONITORING AND HANDLING <strong>OF</strong> <strong>THE</strong> DATA<br />
For assuring the quality of the data an in-house monitoring after receipt of the CRFs will be<br />
conducted. For removing possible ambiguities enquiries will be sent to the investigating<br />
physician.<br />
The original Case Report forms will be numbered consecutively. The number of the report<br />
form will be handed as document/patients number.<br />
All investigational results as well as all deviations of the protocol will be summarised in a<br />
final report.<br />
Page 5 of 6 Version 3: 06.06.2005
IBS: Post marketing surveillance Eucarbon®<br />
F. TRENKA<br />
Please send the electronically finalized CRF of each patient, either per e-mail or by fax to:<br />
11. STATISTICS<br />
Dr. Robert Heinz und Partner GmbH<br />
c/o Dr. Brigitte Monsberger<br />
Kaiserstrasse 84/9<br />
1070 Wien<br />
E-mail: monsberger@heinz-consulting.com<br />
Fax: +43-1-524 61 78-22<br />
Descriptive measures will be used to report the results of this open label post marketing<br />
surveillance. If necessary, confirmatory analyses will be performed (Wilcoxon-Mann-<br />
Whitney test, two sided, alpha = 0.05).<br />
12. CONFIDENTIALITY AND PUBLICATIONS <strong>OF</strong> <strong>THE</strong> RESULTS<br />
All data and information provided have to be treated confidentially. The information<br />
contained in the CRF and protocol is the property of F. TRENKA and is therefore provided to<br />
the investigator in confidence. It is understood that the information will not be disclosed to<br />
others and publications will not be done without prior written approval from F. TRENKA.<br />
3 months after tzhe end of the post marketing surveillance a final report with statistical<br />
analysis and clinical interpretation will be performed.<br />
1<strong>3.</strong> STORAGE<br />
The CRFs will be stored for later use and evaluations 10 years after the surveillance.<br />
14. APPENDICES<br />
Case Report Form (CRF)<br />
Page 6 of 6 Version 3: 06.06.2005