PC Pharma Limited - Colombo Stock Exchange
PC Pharma Limited - Colombo Stock Exchange
PC Pharma Limited - Colombo Stock Exchange
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Introductory Document<br />
for<br />
Listing of 101,000,020 Ordinary Voting Shares of the company<br />
on Diri Savi Board<br />
of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong><br />
The delivery of this Introductory Document shall not under any circumstance constitute a<br />
representation or create any implication or suggestion, that there has been no material<br />
change in the affairs of the Company since the date of this Introductory Document.<br />
If you are in any doubt regarding the contents of this document you should consult your<br />
stockbroker, bank manager, lawyer or any other professional advisor.<br />
This Introductory Document is dated 27 th December 2011<br />
For further inquiries, please contact the Managers to the Introduction<br />
MANAGERS TO THE INTRODUCTION<br />
NAVARA CAPITAL LIMITED<br />
www.navaracapital.com
Responsibility for the Content of the Introductory Document<br />
This Introductory Document has been prepared from information provided by <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
and other publicly available information. The Directors of the Company, collectively and individually,<br />
having made all reasonable inquiries, confirm that to the best of their knowledge and belief, the<br />
information contained herein is true and correct in all material respects and that there are no other<br />
material facts, the omission of which, would make any statement herein misleading.<br />
No person is authorized to give any information or to make any representations not contained in this<br />
Introductory Document and if given or made, any such information or representation must not be<br />
relied upon as having been authorized by the Company.<br />
Copies of the Introductory Document may be obtained from the Manager or any member firm and<br />
trading member firm of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong> as specified in section 2.4 on page 3 titled<br />
“Member Firms and Trading Member Firms of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong>”.<br />
The <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong> (CSE) has taken reasonable care to ensure full and fair disclosure of<br />
information in this Introductory Document. However, the CSE assumes no responsibility for the<br />
accuracy of the statements made, opinions expressed or reports included in this Introductory<br />
Document.
Abbreviations<br />
CBSL Central Bank of Sri Lanka<br />
GDP<br />
Gross Domestic Product<br />
GOSL Government of Sri Lanka<br />
HFA<br />
Mn.<br />
Health For All<br />
Million<br />
MOH Ministry of Health<br />
MSD<br />
N/A<br />
OGL<br />
Rs.<br />
<strong>PC</strong>P<br />
PTF<br />
S<strong>PC</strong><br />
SPMC<br />
WHO<br />
YOY<br />
Glossary of Terms<br />
Medical Supplies Division<br />
Not Applicable<br />
Orient Garments PLC<br />
Sri Lankan Rupees<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Presidential Task Force<br />
State <strong>Pharma</strong>ceutical Corporation<br />
State <strong>Pharma</strong>ceuticals Manufacturing Corporation<br />
World Health Organization<br />
Year on Year<br />
Board/ Board of Directors The Board of Directors of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Company/ The Company <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Ordinary Share(s) Ordinary Voting Share(s) of the Company<br />
Group/ The Group <strong>PC</strong>H Holdings <strong>Limited</strong>
Table of Contents<br />
Abbreviations<br />
Glossary of Terms<br />
Table of Contents<br />
1.<br />
2.<br />
2.1<br />
2.2<br />
2.3<br />
2.4<br />
3.<br />
3.1<br />
3.2<br />
3.3<br />
3.4<br />
3.5<br />
4.<br />
4.1<br />
4.2<br />
4.3<br />
4.4<br />
4.5<br />
4.6<br />
4.7<br />
4.8<br />
4.9<br />
4.10<br />
4.11<br />
4.12<br />
Corporate Information ............................................................................................ 1<br />
Information Relevant to the Introduction ................................................................ 3<br />
Introduction of Ordinary Voting Shares for Listing .......................................................... 3<br />
Copies of the Introductory Document ............................................................................. 3<br />
Managers to the Introduction .......................................................................................... 3<br />
Member Firms and Trading Member Firms of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong> ................. 3<br />
Overview of the Healthcare and <strong>Pharma</strong>ceutical Industry ........................................ 6<br />
Introduction ..................................................................................................................... 6<br />
Sri Lankan Healthcare....................................................................................................... 6<br />
Overview of Health Sector developments in Sri Lanka .................................................... 7<br />
<strong>Pharma</strong>ceutical Industry in Sri Lanka ............................................................................... 9<br />
Impact of the Economic Conditions ............................................................................... 12<br />
Company Profile .................................................................................................... 14<br />
Group Overview ............................................................................................................. 14<br />
Company Overview ........................................................................................................ 15<br />
Milestones ...................................................................................................................... 16<br />
Product Portfolio ............................................................................................................ 17<br />
Suppliers ......................................................................................................................... 18<br />
Distribution .................................................................................................................... 20<br />
Financial Highlights ........................................................................................................ 22<br />
Key Financial Ratios ........................................................................................................ 24<br />
Loans, Overdrafts and Other Borrowings ...................................................................... 25<br />
Dividend Policy and Payments during the Past Three Years.......................................... 25<br />
IT Infrastructure ............................................................................................................. 26<br />
Accreditation and Certifications .................................................................................... 26
5.<br />
5.1<br />
5.2<br />
5.3<br />
5.4<br />
6.<br />
6.1<br />
6.2<br />
7.<br />
7.1<br />
7.2<br />
7.3<br />
8.<br />
8.1<br />
8.2<br />
8.3<br />
8.4<br />
8.5<br />
8.6<br />
9.<br />
10.<br />
11.<br />
12.<br />
Future Strategies and Risk Analysis ........................................................................ 27<br />
Future Strategies ............................................................................................................ 27<br />
Underlying Assumptions on Which Future Strategies Are Based on ............................. 28<br />
Market Approach ........................................................................................................... 28<br />
Associated Risks Related to Future Strategies ............................................................... 29<br />
Human Resources .................................................................................................. 31<br />
Staff Strength ................................................................................................................. 31<br />
Corporate Management Team ....................................................................................... 33<br />
Corporate Structure .............................................................................................. 35<br />
Share Capital Structure .................................................................................................. 35<br />
Share Capital .................................................................................................................. 36<br />
Shareholding Structure .................................................................................................. 37<br />
Board of Directors ................................................................................................. 39<br />
Profile of Directors ......................................................................................................... 40<br />
Directors’ Interest in Shares ........................................................................................... 41<br />
Statement – Chairman/ Chief Executive Officer ............................................................ 41<br />
Statement – Board of Directors ..................................................................................... 41<br />
Director’s Emoluments................................................................................................... 42<br />
Other Disclosures ........................................................................................................... 42<br />
Other Information Relating to the Company .......................................................... 43<br />
Corporate Governance Practices ............................................................................ 44<br />
Statutory Declaration ............................................................................................ 46<br />
Statutory and Other General Information .............................................................. 47<br />
Annexure I - Auditors Report and Financial Statements as at March 31, 2009 ................... 49<br />
Annexure II - Auditors Report and Financial Statements as at March 31, 2010 .................. 64<br />
Annexure III - Auditors Report and Financial Statements as at March 31, 2011 ................. 79<br />
Annexure IV - Interim Financial Statements as at September 30, 2011 .............................. 97
1.<br />
Corporate Information<br />
Company Name<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Legal Form<br />
The company was incorporated in Sri Lanka on 5 th of May 2006 as a private limited<br />
liability company under the Companies Act No. 17 of 1982 and reregistered under the<br />
Companies Act No. 07 of 2007 on 1 st of August 2008. Subsequently converted to a<br />
public limited liability company on 24 th of October 2011.<br />
Company Registration Number<br />
PV 528 PB<br />
Main line of business<br />
Import, supply and distribution of <strong>Pharma</strong>ceuticals, Medical Devices, Nutraceuticals<br />
and Medical Consumables.<br />
Registered Office<br />
No. 451<br />
Galle Road<br />
<strong>Colombo</strong> 3.<br />
Business Office<br />
No. 451<br />
Galle Road<br />
<strong>Colombo</strong> 3.<br />
Tel: +94 11 4724242 Fax: +94 11 5373617<br />
Email: info@pcpharma.lk<br />
Website: www.pcpharma.lk<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 1
Board of Directors<br />
Mr. Saheedul Hijiry Mohamed Rishan - Chairman<br />
Mrs. Sithy Sharmila Rishan - Non-Executive Director<br />
Mr. Mushtaq Ahmed Ikram - Director<br />
Mr. Ramasamy Selvaraj - Non-Executive Independent Director<br />
Mr. Prasanna Lakshantha Wijesekara - Non-Executive Director<br />
Mrs. Shanthi Sri Nanda Goonaratne - Non- Executive Independent Director<br />
Company Secretary<br />
Seccom (Private) <strong>Limited</strong><br />
No. 1E 2/1, De Fonseka Place<br />
<strong>Colombo</strong> 5<br />
Sri Lanka<br />
Tel: +94 11 2590176, Fax: +94 11 2581618<br />
Company Auditors<br />
SJMS Associates<br />
Chartered Accountants<br />
2, Castle Lane<br />
<strong>Colombo</strong> 4<br />
Sri Lanka<br />
Tel: +94 11 2580409, Fax: +94 11 2582452<br />
Bankers to the Company<br />
National Development Bank PLC<br />
103 A, Dharmapala Mawatha<br />
<strong>Colombo</strong> 07<br />
Commercial Bank of Ceylon PLC<br />
Foreign Branch<br />
21, Bristol Street<br />
<strong>Colombo</strong> 01<br />
Company Lawyer<br />
FJ & G De Seram<br />
Attorney-at-Law<br />
216, De Saram Place<br />
<strong>Colombo</strong> 10<br />
Tel: +94 11 4605100, Fax: +94 11 4718220<br />
2 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
2.<br />
2.1<br />
2.2<br />
2.3<br />
2.4<br />
Information Relevant to the Introduction<br />
Introduction of Ordinary Voting Shares for Listing<br />
This Introductory Document dated 27 th December 2011 is published for the purpose of<br />
obtaining a listing on the Diri Savi Board of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong> for the<br />
Ordinary Voting Shares of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>.<br />
Copies of the Introductory Document<br />
Copies of the Introductory Document can be obtained from the Managers to the<br />
Introduction or members and trading members of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong>.<br />
Managers to the Introduction<br />
Navara Capital <strong>Limited</strong><br />
No. 12 B, Gregory’s Road<br />
<strong>Colombo</strong> 07<br />
Sri Lanka.<br />
Tel: +94 11 4378387, Fax: +94 11 2698524<br />
E-mail: info@navaracapital.com<br />
Website: www.navaracapital.com<br />
Member Firms and Trading Member Firms of the <strong>Colombo</strong> <strong>Stock</strong> <strong>Exchange</strong><br />
Acuity <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
Level 6, Acuity House<br />
53, Dharmapala Mawatha<br />
<strong>Colombo</strong> 03.<br />
Tel: 2206206<br />
Fax: 2206298/9<br />
E-mail: sales@acuitystockbrokers.com<br />
Bartleet Religare Securities (Pvt) <strong>Limited</strong><br />
Level "G", Bartleet House<br />
65, Braybrooke Place<br />
<strong>Colombo</strong> 02.<br />
Tel: 5220200<br />
Fax: 2434985<br />
E-mail: info@bartleetstock.com<br />
Asia Securities (Pvt) <strong>Limited</strong><br />
Level 21, West Tower<br />
World Trade Center<br />
Echelon Square<br />
<strong>Colombo</strong> 01.<br />
Tel: 2423905, 5320000<br />
Fax: 2336018<br />
E-mail: enquiry@asiacapital.lk<br />
Capital Trust Securities (Pvt) <strong>Limited</strong><br />
42, Mohamed Macan Marker Mawatha<br />
<strong>Colombo</strong> 03.<br />
Tel: 5335225<br />
Fax: 5365725<br />
E-mail: inquiries@capitaltrust.lk<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 3
CT Smith <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
4-14, Majestic City<br />
10, Station Road<br />
<strong>Colombo</strong> 04.<br />
Tel: 2552290-4<br />
Fax: 2552289<br />
E-mail: ctssales@sltnet.lk<br />
Nation Lanka Equities (Pvt) <strong>Limited</strong><br />
Level 9 - Ceylinco House<br />
69, Janadhipathi Mawatha<br />
<strong>Colombo</strong> 01.<br />
Tel: 4714300, 4714388, 4714389<br />
Fax: 2387228<br />
E-mail: info@nlequities.com<br />
John Keells <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
130, Glennie Street<br />
<strong>Colombo</strong> 02.<br />
Tel: 2306250, 2338066-7<br />
Fax: 2326863, 2342068<br />
E-mail: jkstock@keells.com<br />
NDB <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
5th Floor, NDB Building<br />
40, Navam Mawatha<br />
<strong>Colombo</strong> 02.<br />
Tel : 2314170-8<br />
Fax: 2314180<br />
E-mail: mail@ndbs.lk<br />
Somerville <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
137, Vauxhall Street<br />
<strong>Colombo</strong> 02.<br />
Tel: 2329201-5, 2332827, 2338292-3<br />
Fax: 2338291<br />
E-mail: ssb-web@sltnet.lk<br />
Heraymila Securities <strong>Limited</strong><br />
Level 8, South Wing<br />
Millennium House<br />
46/58 Nawam Mawatha<br />
<strong>Colombo</strong> 02<br />
Tel: 2359100, Fax: 2305522<br />
D N H Financial (Pvt) <strong>Limited</strong><br />
Level 16, West Tower<br />
World Trade Centre<br />
<strong>Colombo</strong> 01.<br />
Tel: 5700777<br />
Fax: 5736264<br />
E-mail: info@dnhfinancial.com<br />
J B Securities (Pvt) <strong>Limited</strong><br />
150, St. Joseph Street<br />
<strong>Colombo</strong> 14.<br />
Tel: 2490900, 0772490900<br />
Fax: 2430070, 2446085, 2447875<br />
E-mail: jbs@jb.lk<br />
Lanka Securities (Pvt) <strong>Limited</strong><br />
228/2, Galle Road<br />
<strong>Colombo</strong> 04.<br />
Tel: 4706757, 2554942<br />
Fax: 4706767<br />
E-mail: lankasec@sltnet.lk<br />
SC Securities (Pvt) <strong>Limited</strong><br />
2nd Floor<br />
55, D.R. Wijewardena Mawatha<br />
<strong>Colombo</strong> 10.<br />
Tel : 4711000, Fax: 2394405<br />
E-mail: cscres@sltnet.lk<br />
Capital Alliance Securities (Pvt) <strong>Limited</strong><br />
Level 5, "Millennium House"<br />
46/58 Navam Mawatha<br />
<strong>Colombo</strong> 02<br />
Tel: 2317777, Fax: 2317788<br />
E-mail: general@capitalalliance.lk<br />
First Guardian Equities (Pvt) <strong>Limited</strong><br />
32nd Floor, East Tower,<br />
World Trade Centre, Echelon Square<br />
<strong>Colombo</strong> 01<br />
Tel: 5884400<br />
Fax: 5884401<br />
4 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
E-mail: info-hasl@heraymila.com E-mail: info@firstguardianequities.com<br />
SKM Lanka Holdings (Pvt) <strong>Limited</strong><br />
377/3, Galle Road<br />
<strong>Colombo</strong> 03<br />
Tel: 2372413-5<br />
Fax: 2372416<br />
E-mail: info@skmlankaholdings.com<br />
IIFL Securities Ceylon (Pvt) <strong>Limited</strong><br />
27th Floor, East Tower<br />
World Trade Center, Echelon Square<br />
<strong>Colombo</strong> 01.<br />
Tel. 011 2333000, Fax: 011 2333383<br />
E-mail: info.ceylon@iiflcap.com<br />
Richard Pieris Securities (Pvt) <strong>Limited</strong><br />
69, Hyde Park Corner<br />
<strong>Colombo</strong> 02.<br />
Tel: 7448900, Fax: 2675064<br />
E-mail: jayantha@rpsecurities.com<br />
New World Securities (Pvt) <strong>Limited</strong><br />
2nd Floor, 45/2,<br />
Braybrooke Street<br />
<strong>Colombo</strong> 2.<br />
Tel: 2358700/20, Fax: 2358701<br />
E-mail: info@nws.lk<br />
Asha Phillip Securities <strong>Limited</strong><br />
Level 4, "Millennium House"<br />
46/58, Navam Mawatha, <strong>Colombo</strong> 2.<br />
Tel. 2429100<br />
Fax: 2429199<br />
E-mail: apsl@ashaphillip.net<br />
Taprobane Securities (Pvt) Ltd.<br />
2nd Floor, No. 10, Gothami Road<br />
<strong>Colombo</strong> 08.<br />
Tel: 5328200<br />
Fax: 5328277<br />
E-mail: info@taprobane.lk<br />
SMB Securities (Pvt) <strong>Limited</strong><br />
47, Dharmapala Mawatha<br />
<strong>Colombo</strong> 03<br />
Tel: 5232091<br />
Fax: 5339292<br />
E-mail: admin@smbsecurities.lk<br />
TKS Securities (Pvt) <strong>Limited</strong><br />
19-01, East Tower<br />
World Trade Centre, Echelon Square<br />
<strong>Colombo</strong> 1.<br />
Tel: 7857799, Fax: 7857857<br />
E-mail: ralph@tks.lk<br />
Claridge <strong>Stock</strong>brokers (Pvt) <strong>Limited</strong><br />
No.10, Gnanartha Pradeepa Mawatha<br />
<strong>Colombo</strong> 08<br />
Tel: 2697974, Fax: 2677576<br />
E-mail: fonseka@mackwoods.com<br />
Arrenga Capital (Pvt) <strong>Limited</strong><br />
Level 23, East Tower<br />
World Trade Centre, Echelon Square<br />
<strong>Colombo</strong> 1<br />
Tel: 7277000 to 98, Fax: 7277099<br />
E-mail: dihand@arrengacapital.com<br />
Assetline Securities (Pvt) Ltd<br />
No. 282, Kaduwela Road<br />
Battaramulla.<br />
Tel. 4700111, 2307366<br />
Fax: 4700112, 2307365<br />
E-mail:dpgs1@sltnet.lk<br />
LOLC Securities <strong>Limited</strong><br />
Level 18, West Tower,<br />
World Trade Centre, <strong>Colombo</strong> 1.<br />
Tel: 7880880<br />
Fax: 2434771<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 5
3.<br />
3.1<br />
3.2<br />
Overview of the Healthcare and <strong>Pharma</strong>ceutical Industry<br />
Introduction<br />
Development of the Sri Lankan Healthcare and <strong>Pharma</strong>ceuticals Industry to its current form<br />
can be traced to the introduction of the Sri Lanka National <strong>Pharma</strong>ceutical Policy in 1970.<br />
The policy recognized the need for attention and support for the Healthcare and<br />
<strong>Pharma</strong>ceuticals Industry and paved the way to allow customers and healthcare providers<br />
access to good quality pharmaceuticals at least possible prices.<br />
Amid strong regulations the private sector continued to expand giving the patients access to<br />
high quality pharmaceuticals at their convenience. This has contributed immensely to Sri<br />
Lanka achieving the best health indices in the South Asian region.<br />
Sri Lankan Healthcare<br />
Post independent Sri Lanka has a long history of the government taking complete<br />
responsibility for the provision of healthcare to the community, and remained a wellestablished<br />
policy of all successive governments in the post-independence era.<br />
Over the years the government of Sri Lanka (GOSL) has taken several steps in the<br />
development of the healthcare and pharmaceuticals industry. The National <strong>Pharma</strong>ceutical<br />
Policy was implemented in 1970 with the aim of ensuring that people get good quality<br />
pharmaceuticals at the lowest possible prices.<br />
In 1980 the GOSL endorsed the goal of “Health for all by the year 2000” (HFA 2000) with<br />
primary healthcare as its focus. To further facilitate achieving HFA 2000, greater emphasis<br />
was placed on decentralization of health administration. Priority was given to the<br />
identification of primary healthcare components and development of an implementation<br />
model for application on a national scale.<br />
As a result, existing large hospitals were to provide general and specialized curative services.<br />
While the secondary care hospitals, divisional health centers and sub divisional health<br />
centers were established by upgrading the existing medical institutions.<br />
In 1992, a Presidential Task Force (PTF) was appointed to formulate a National Health Policy.<br />
The major thrust of the policy was health promotion, prevention and control of disease<br />
fostering of healthy life styles, human resource development, strengthening the quality and<br />
range of existing health services, and decentralization of health administration. Similarly in<br />
1997 a Health Sector Reforms Implementation Unit was established in the Ministry of Health<br />
to oversee the implementation of the proposals of the PTF. In 2002, action was initiated to<br />
develop a Health Master Plan with wide stakeholder involvement to address measures of<br />
restructuring “the system” as well as necessary interventions for health service delivery<br />
targeting the year 2016, and to identify, prioritize and anchor programmes/projects.<br />
6 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
3.3<br />
GOSL in its continuous efforts in improving the level of basic health had increased the<br />
expenditure on national healthcare by more than 60% since 2006 from Rs. 44.1 Bn.(2006) to<br />
Rs. 70.8 Bn. (planned for 2011).<br />
Figure 3.1: GOSL Healthcare Expenditure 2004-2011<br />
Millions<br />
Rs. 100,000<br />
Rs. 90,000<br />
Rs. 80,000<br />
Rs. 70,000<br />
Rs. 60,000<br />
Rs. 50,000<br />
Rs. 40,000<br />
Rs. 30,000<br />
Rs. 20,000<br />
Rs. 10,000<br />
Rs. 0<br />
Source: CBSL Annual Report<br />
2004 2005 2006 2007 2008 2009 2010<br />
Prov.<br />
Overview of Health Sector developments in Sri Lanka<br />
2011<br />
App.<br />
Current<br />
Expenditure<br />
Capital<br />
Expenditure<br />
Total<br />
Expenditure<br />
Further, it has been recognized that, the improvement of the nutritional status of people<br />
should be a coordinated effort of all the stakeholders such as Ministry of Health, Doctors,<br />
Nurses, patients, General public, <strong>Pharma</strong>ceutical industry and all other relevant parties.<br />
Malnutrition, re-emergence of certain communicable diseases, a rising trend in Non-<br />
Communicable Diseases (NCDs) are highlighted areas where the Sri Lankan health sector<br />
should be improved in future to address these issues while arranging mass scale national<br />
awareness campaign to change the lifestyle of the people and to discourage unhealthy food<br />
patterns.<br />
The Ministry of Health continues to carry out several programmes to further improve<br />
healthcare delivery nationally with special emphasis on the newly liberated Northern and<br />
Eastern provinces. Health sector infrastructure including human resources has increased<br />
when compared to the year 2009 as stated below;<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 7
Table 3.1: Health Sector Infrastructure Including Human Resource<br />
Item 2007 2008 2009 2010 (a)<br />
Government<br />
Hospitals (practicing western medicine) (No.) 619 619 555 568<br />
Beds (No.) 62,749 65,835 68,897 69,501<br />
Primary Healthcare Units (No.) 387 411 475 476<br />
Doctors (No.) 11,442 13,026 13,633 14,125 (b)<br />
Assistant medical practitioners (No.) 1,244 1,229 1,198 1,158<br />
Nurses (No.) 22,088 22,996 25,549 27,494<br />
Attendants (No.) 7,201 7,184 8,301 8,189<br />
Total government expenditure on health (Rs.Bn) 68.7 74.5 71.5 73.8<br />
Current expenditure (Rs.Bn) 51.7 55.9 58.8 60.5<br />
(a) Provisional<br />
Source: CBSL Annual Report<br />
(b) Including Intern<br />
Medical Officers<br />
In Sri Lanka, health care expenditure structure consists of the following expenditure<br />
schemes as depicted below<br />
Figure 3.2: Healthcare Expenditure Scheme<br />
44%<br />
Source: CBSL Annual Report<br />
Healthcare Expediture Schemes<br />
0%<br />
2%<br />
5%<br />
49%<br />
Government Budget<br />
Voluntary health insurance<br />
schemes<br />
Social health insurance<br />
Out-of-pocket payments /<br />
Household expenditure<br />
Other<br />
8 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
3.4<br />
<strong>Pharma</strong>ceutical Industry in Sri Lanka<br />
Sri Lanka National <strong>Pharma</strong>ceutical Policy<br />
Sri Lanka National <strong>Pharma</strong>ceutical Policy was established in 1970 following a report<br />
submitted by Dr. S. A. Wickramasinghe and Prof. Senaka Bibile with the aim of ensuring<br />
that people get good quality pharmaceuticals at the lowest possible prices.<br />
Under the integrated National <strong>Pharma</strong>ceutical Policy a central procuring agency of<br />
pharmaceuticals, calling for worldwide bulk tenders were established. The pharmaceuticals<br />
purchased were limited to the approved list of the national formulary. The public health<br />
sector was compelled to obtain all their requirements from the central buying agency. This<br />
policy was watered down by the subsequent governments by permitting the private sector<br />
importation of multiple brands.<br />
Introduction of the National <strong>Pharma</strong>ceutical Policy led to the establishment of the State<br />
<strong>Pharma</strong>ceutical Corporation in 1971 this resulted in the dominance of the multinational<br />
corporations on the drug trade being successfully broken as they were made to compete<br />
with each other and with generic drug producers, enabling the people to obtain<br />
pharmaceuticals much cheaper. Branded pharmaceuticals were replaced to a certain<br />
extent by generic pharmaceuticals in the prescription and sale of medicines. According to<br />
the Chairman of the Sri Lanka Chamber of <strong>Pharma</strong>ceutical Industry approximately 10% of<br />
the country demand for medicine is being met by local manufacturers with the balance<br />
being imported. Over the years the number of imported pharmaceuticals has increased<br />
significantly. By the year 2000 Sri Lanka had approximately 9000 registered medicinal<br />
pharmaceuticals, hundreds of which were non-essential or highly expensive or even<br />
dangerous.<br />
In 2005 the National Medicinal Drugs Policy (NMDP) was established with the aim of<br />
cutting down on drug expenses and getting quality pharmaceuticals at affordable prices.<br />
Implementation of the NMDP reduced the number of pharmaceuticals imported,<br />
prescribed and sold in Sri Lanka to about 350 varieties.<br />
Cosmetic Devices and Drug Authority (CDDA)<br />
All <strong>Pharma</strong>ceutical and medical equipment imports to Sri Lanka are stringently monitored<br />
by the drug regulatory arm of the MOH, the Cosmetic Devices and Drug Authority (CDDA).<br />
The CDDA ensures that the quality standards of pharmaceuticals and medical devices<br />
available to the Sri Lankan market meet the standards set by the authority consistently.<br />
Post surveillance procedures ensures that all those responsible for holding and distributing<br />
medical products imported to the country are held responsible until such time the products<br />
are delivered to the end user for consumption.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 9
Guidelines are in place making it mandatory for <strong>Pharma</strong>ceutical and medical equipment<br />
importers to register the manufacturing company and the individual medical items to be<br />
imported to the country with the CDDA. Only items that meet the approval of the CDDA<br />
receive a certificate of registration in testimony of approved quality and an import license<br />
that grants the importer permission to import the given item.<br />
The CDDA insists upon WHO GMP (Good Manufacturing Practices) certification for <strong>Pharma</strong><br />
manufacturing facilities in order to establish acceptability of manufacturing standards.<br />
Similarly the “Free Sales Certificates” (FSC) is mandatory to establish acceptability of<br />
standards associated with the production of medical devices.<br />
In addition, the importer and the storage facilities too need to be registered with the MOH<br />
and registration renewed after inspection, on an annual basis.<br />
While the government has increased the level of restrictions and laws to ensure the safety<br />
of the pharmaceuticals distributed, the pharmaceutical industry has grown significantly<br />
over the years.<br />
Based on reports by IMS Health an independent research unit that specializes in<br />
healthcare, the Sri Lankan government spends approximately US $ 140 million per year on<br />
medicine alone in government hospitals from outpatient treatment to serious surgeries<br />
which takes the total annual government expenditure on healthcare to more than US $ 700<br />
million annually.<br />
IMS Health audited retail market for Sri Lanka grew by 16.2% in the second quareter of<br />
2010 to reach US $ 175 million As there are only six significant local manufacturers<br />
producing an estimated 10% of the requirement the market is mainly dependent upon<br />
imported products. Markets such as chronic care, cardiovascular and anti-diabetics are all<br />
driving growth.<br />
Furthermore, IMS estimates that the market for originator brands have grown by 16% by<br />
the second quarter of 2010 which has been lately outpaced by generics which have grown<br />
at a rate of 19%. Generics markets share has increased significantly over the recent years<br />
and currently consists of almost two thirds of the total market.<br />
According to the Cabinet Spokesman the Sri Lankan market for over the counter medicine<br />
(OTCs) is worth about Rs. 40 Bn and top 100 products contributes 37% to the retail market<br />
by value. The market is expected to grow at an added annual growth rate of 15% for the<br />
next four years. The government is keen to widen the availability of OTCs island wide as<br />
well as reduce the cost of the pharmaceuticals.<br />
10 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
The Medical Supplies Division<br />
The Medical Supplies Division (MSD) of Ministry of Health of Sri Lanka is the main central<br />
organization responsible for providing all <strong>Pharma</strong>ceuticals, Surgical items, Laboratory<br />
Items, Radioactive Items and Printed materials etc. needed by Hospitals and Healthcare<br />
Institutions throughout the country. The main functions of MSD are estimating, indenting,<br />
procuring, storing, monitoring, distributing and accounting of medical supplies to all<br />
government health institutions in Sri Lanka. In addition MSD is responsible for supplying<br />
the private sector with all dangerous pharmaceuticals and essential medical items which<br />
are not available in the open market.<br />
MSD indents total requirements of medical items mainly through State <strong>Pharma</strong>ceutical<br />
Corporation (S<strong>PC</strong>) which is the procurement agent for MSD. In addition MSD has its own<br />
supply branch for emergency local purchases and selected items on regular basis from<br />
overseas and local sources. Furthermore MSD receives and distributes donations from<br />
donor agencies such as WHO/UNICEF and others.<br />
MSD has 18 Bulk warehouses at its main building, 3 Bulk warehouses at Angoda, 5 bulk<br />
warehouses at Wellawatta, one warehouse at Digana and one warehouse at Welisara to<br />
receive, store and issue these items.<br />
MSD is responsible for the distribution of medical items directly to the 26 Regional<br />
Medicine Supplies Divisions and 50 major hospitals and healthcare institutions<br />
administered by Central Government. These 26 Regional Medical Supplies Divisions (RMSD)<br />
are responsible for the supply of medical items to General Hospitals, Base hospitals,<br />
District Hospitals, Peripheral Units, Rural Hospitals, Central Dispensaries, Maternity Homes<br />
& Central Dispensaries and other small Hospitals under the purview of Provincial Councils.<br />
The State <strong>Pharma</strong>ceutical Corporation<br />
The State <strong>Pharma</strong>ceuticals Corporation (S<strong>PC</strong>) was established in 1971 under the State<br />
Industrial Corporations Act No. 49 of 1957.<br />
S<strong>PC</strong> was the sole supplier of pharmaceuticals, surgical consumable items, laboratory<br />
chemicals and equipment mainly to the MSD and all institutions administered by the health<br />
ministry.<br />
In 1977, with the advent of the open economy S<strong>PC</strong> was called upon to compete with the<br />
private sector. Even though the monopoly status ceased, S<strong>PC</strong> continued to operate as a<br />
viable institution, continuing to hold about 30% of the private sector market share – which<br />
is the largest share for any single pharmaceutical firm. At present about 2000<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 11
3.5<br />
pharmaceuticals covering a wide spectrum of pharmacological activity are imported by the<br />
S<strong>PC</strong>.<br />
The well-established island wide network of distribution of the S<strong>PC</strong> ensures that products<br />
reach every part of the country. S<strong>PC</strong> has 18 Rajya Osu Salas, 36 Franchise Osu Salas, 48<br />
Distributors and 20 Authorized Retailers spread throughout the country.<br />
The Rajya Osu Salas and Franchise Osu Salas maintain the highest standards in pharmacy<br />
and dispensing practice. The newly opened Quality Assurance Laboratory at the Head<br />
Office carries out the quality testing of all the pharmaceuticals.<br />
The S<strong>PC</strong> is the largest buying institution of <strong>Pharma</strong>ceutical and medical products in the<br />
country. The purchasing is done locally or through global tenders.<br />
The State <strong>Pharma</strong>ceutical and Manufacturing Corporation<br />
SPMC is a subsidiary of S<strong>PC</strong> and operate a state of art 50,000 sq. ft. pharmaceutical<br />
manufacturing facility. All products are produced exclusively for the S<strong>PC</strong>. The facility is<br />
equipped with modern equipment with an annual installed capacity of 550 Mn. Units of<br />
tablets, capsules and 60,000 liters of dry syrup. The present production utilizes installed<br />
capacity by about 95% and is expected to increase further.<br />
SPMC products are ‘braded generics’ and all tablets, capsules and labels carry the letter<br />
SPMC.<br />
Impact of the Economic Conditions<br />
The pharmaceutical and healthcare industry has historically been relatively immune to<br />
economic fluctuation, although at times of recessions the industry experiences the reduced<br />
investments and research funding.<br />
Over the years, the reliance of Sri Lankan pharmaceutical market on UK and USA gradually<br />
transferred towards Asian countries such as India, Bangladesh, China and Korea. This<br />
resulted in lower cost of the pharmaceuticals that are being imported and less exposure to<br />
the volatilities of the economic crisis experienced in the west during the last few years.<br />
Despite all other external factors Analysts and industry players remain optimistic about the<br />
Sri Lankan market due to the expanded opportunities in the Northern and Eastern<br />
Provinces and due to projected growth in therapeutics and nutraceuticals.<br />
Continued increase in government expenditure will result in an increase in growth of the<br />
healthcare industry allowing pharmaceutical companies access to new geographical areas.<br />
Due to the insufficient capacity of public healthcare system the private healthcare market<br />
has been experiencing a rapid growth in the recent past with some companies growing by<br />
more than 25% YOY according to the latest annual reports.<br />
12 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
On 19 th October 2011 Cabinet Approval was given to establish an industrial zone in<br />
Kurunegala exclusively for investments in pharmaceutical companies for the production of<br />
most commonly used pharmaceuticals and medical equipment. According to media reports<br />
over 10 reputed pharmaceutical manufacturers have expressed their willingness to invest<br />
in this zone. Post war Sri Lanka has also given rise to increase in demand for<br />
pharmaceuticals and medical equipment. According to the Business Monitor International<br />
which analyses country risk across 175 countries, the projected expenditure for Lankan<br />
<strong>Pharma</strong>ceuticals for 2011 is expected to increase by 15% (to US $ 444 Mn) in comparison to<br />
2010 and Sri Lanka is the region's 14 th most attractive market, behind Vietnam and ahead<br />
of Bangladesh. Sri Lanka scores above the regional average for country risk, given its<br />
relatively stable business operating environment. Although Sri Lanka is an emerging<br />
pharmaceutical market, it boasts relatively well developed healthcare infrastructure and<br />
coverage. The private sector plays an increasingly important role in healthcare and the<br />
stimulation for the growth of the <strong>Pharma</strong>ceuticals market.<br />
The shift of sourcing destinations towards low cost manufacturing countries has not<br />
affected the attempt to accomplish the required standards and quality expectations. While<br />
the world’s largest producers continue to operate from developed countries, over the<br />
years some of the world’s leading pharmaceutical manufacturers and suppliers have<br />
shifted their sourcing towards low cost countries such as India, China, Korea and<br />
Bangladesh etc. to reduce the impact of economic fluctuations experienced in the west.<br />
The Sri Lankan <strong>Pharma</strong>ceutical Industry has been consistently successful in deriving the<br />
maximum dividends from Socio-economic development opportunities in the country. Also<br />
<strong>Pharma</strong>ceutical Companies have been successful in overcoming the hurdles which surfaced<br />
from time to time such as changes in the National Medicinal and Drugs Policy (NMDP,)<br />
restrictions on importation of certain pharmaceuticals, economic situation in the country<br />
and currency depreciation.<br />
The market remains very attractive with the growing GDP which leads to higher disposable<br />
income which in turn will be an opportunity for the nutraceuticals market to further<br />
develop. The increasing awareness among Sri Lankans about health and wellbeing will<br />
increase demand for products in this sector which will continue to grow at very high rates.<br />
The unassailable economic growth, political stability and proactive measures taken by the<br />
government, argue well for the future of the <strong>Pharma</strong>ceutical Industry in Sri Lanka.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 13
4.<br />
4.1<br />
Company Profile<br />
Group Overview<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is part if the <strong>PC</strong>H Group of Companies which operate under the<br />
guidance of the group Chairman Mr. S. H. M. Rishan who is also the principle shareholder.<br />
<strong>PC</strong>H group consists of <strong>PC</strong>H Holdings <strong>Limited</strong>, <strong>PC</strong> House PLC, <strong>PC</strong> <strong>Pharma</strong> LTD and Orient<br />
Garments PLC. <strong>PC</strong>H Holdings <strong>Limited</strong> owns 86.62% of <strong>PC</strong> <strong>Pharma</strong> LTD.<br />
<strong>PC</strong>H Holdings <strong>Limited</strong><br />
<strong>PC</strong>H Holdings <strong>Limited</strong> mainly focuses on capitalizing on profitable investments, related and<br />
non-related investments that capitalizes profitable ventures with vigil and market<br />
intelligence. The company maintains close liaison with business partners and investors and<br />
initiates discussions for strategic business and equity investment. <strong>PC</strong>H Holdings <strong>Limited</strong> is<br />
also equipped with a market intelligence arm which closely monitors a few selected<br />
industries on the opportunities available for investment which will generate high returns.<br />
<strong>PC</strong> House PLC<br />
<strong>PC</strong> House PLC in partnership with more than 35 reputed manufacturers and over 12,000<br />
products is at the forefront of Sri Lanka’s ICT industry providing computer hardware and<br />
software solutions. The Company is a preferred supplier for public and private sector<br />
organizations with an annual turnover in excess of Rs. 3.7 Bn for the financial year ended<br />
31 st March 2011. Their strong island-wide distribution network encompasses a wide range<br />
of users across the country, leveraging on more than 37 strategically located branches<br />
island wide. <strong>PC</strong> House is credited with penetrating rural markets with affordable computer<br />
hardware, thereby attempting to bridge the urban-rural digital gap.<br />
The <strong>PC</strong> House PLC Group consists of a workforce of about 600 employees and has now<br />
forayed into the integrated solutions arena with its wholly-owned subsidiary Greenwich<br />
Lanka (Private) <strong>Limited</strong>, which is engaged in system integration and designing IT solutions.<br />
<strong>PC</strong>H has expanded its expertise further, with the setting up of another subsidiary,<br />
Procifinity <strong>Limited</strong>, to engage in Business Process Outsourcing and Knowledge Process<br />
Outsourcing activities, widely seen as one of the future ICT growth areas in Sri Lanka. In<br />
September 2011 <strong>PC</strong> House PLC acquired 90% of Info Serve Pvt <strong>Limited</strong> a company involved<br />
in online research. Chairman Mr. S. H. M. Rishan holds 55.17% stake in <strong>PC</strong> House PLC as at<br />
30 th September 2011.<br />
Orient Garments PLC<br />
As at 30 th September 2011 21.05% of OGL is owned by <strong>PC</strong>H Holdings <strong>Limited</strong> while Mr. S. H.<br />
M. Rishan holds 30% of the shares. OGL is involved in manufacturing and exporting of highend<br />
apparel and specialize in outerwear, casualwear and sportswear. OGL has expertise in<br />
14 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
4.2<br />
apparel for over 28 years and their rich experience in manufacturing and exporting have<br />
made them one of the most reliable clothing suppliers in Sri Lanka.<br />
Orient Garments PLC consists of 5 fully equipped modern production factory units which<br />
have a monthly production capacity of about 300,000 units and a total workforce of about<br />
3,300 employees which contribute towards an annual turnover in excess of Rs. 3.5 Billion.<br />
The business focus of OGL is designing, manufacturing and exporting a wide range of<br />
garments catering to the needs of leading international fashion brands and retailers such as<br />
NEXT, Tesco, Tommy Hilfiger, Polo Ralph Lauren and Burberry.<br />
Structure of <strong>PC</strong>H Group of Companies<br />
55.17%<br />
<strong>PC</strong> House PLC<br />
Company Overview<br />
S. H. M. Rishan<br />
99.90%<br />
<strong>PC</strong>H Holdings <strong>Limited</strong><br />
86.62%<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 15<br />
30%<br />
21.05% Orient Garments PLC<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> which is a subsidiary of <strong>PC</strong>H Holdings <strong>Limited</strong> was incorporated on 5 th<br />
May 2006. During its short tenure in the industry the company has been able to establish<br />
an excellent reputation amongst the health sector professionals in Sri Lanka by offering a<br />
diverse product range at competitive prices supported by superior services.<br />
The main line of business of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> consists of importing, distributing and<br />
marketing a spectrum of pharmaceuticals, medical devices, nutraceuticals and medical<br />
consumables in Sri Lanka. The company represents internationally reputed and established<br />
brands in the respective arena such as Square <strong>Pharma</strong>ceuticals Ltd – Bangladesh, Bbraun<br />
Dialysis Products – Germany, Celia infant and maternal nutrition – Lactalis International<br />
France, EZ Smart Blood Glucose Monitoring System – Tyson Bioresearch Inc. Taiwan and<br />
Aurochem <strong>Pharma</strong>ceuticals Pvt Ltd – India.
4.3<br />
<strong>Pharma</strong>ceutical items of superior quality and repute are sourced from across the globe for<br />
promoting better health and improving the quality of life of the Sri Lankan people.<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>’s keen participation in the pharmaceutical market has carved the<br />
company a niche in the industry, establishing a firm platform from which to develop further<br />
in the direction of the company’s vision to emerge as the prime pharmaceutical importer in<br />
the country.<br />
The Vision<br />
Be an ethical leader in shaping healthy lives in the country.<br />
Mission<br />
� To assume responsibility for the marketing of efficient health products and value<br />
added services preserving corporate integrity, long term independence and<br />
concern for people.<br />
� To provide quality, innovative solutions that support better health and improved<br />
quality of life.<br />
� To be consistently oriented towards the needs of our customers and business<br />
partners, providing superior benefits and creating value that exceeds all our<br />
stakeholder expectations.<br />
� To maintain a corporate culture that encourages professionalism and ethical<br />
business practice.<br />
� To take part in the achievements and dedication of our employees, who are the<br />
pillars of the Company and has continued success.<br />
Milestones<br />
2006<br />
Establishment of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as a private limited liability for the supply of<br />
<strong>Pharma</strong>ceuticals and Medical Devices registered under the Ministry of Health. Concurrently<br />
the direct distribution network of <strong>PC</strong>P and several distribution agreements were<br />
established with Asia Connection, Taiwan and T-Man <strong>Pharma</strong>, Thailand.<br />
2007<br />
<strong>PC</strong>P obtained the government tender for EZ Smart Blood Glucose Monitoring System<br />
during the year Square <strong>Pharma</strong>ceuticals – Bangladesh and Aurochem <strong>Pharma</strong>ceuticals<br />
Products were also launched in Sri Lanka by the company. An anti-cancer products range<br />
was also launched in the same year.<br />
2008<br />
In 2008 the Company became the first <strong>Pharma</strong>ceutical supplier to obtain ISO 9001-2000<br />
accreditation in order to facilitate the rapid growth of the company expansion of direct and<br />
indirect distribution was also carried out which was followed by the launch of the<br />
corporate website. <strong>PC</strong>P also obtained the memberships of the Sri Lanka Chamber of<br />
<strong>Pharma</strong>ceutical Industry (SLCPI) and <strong>Pharma</strong> Promoters’ Association (PPA).<br />
16 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
4.4<br />
2009<br />
The product range was expanded with the launch of renal care range from B|BRAUN<br />
Medical Industries of Germany, Diliner ® DR Capsules – Market leader brand for Diabetic<br />
Peripheral Neuropathic Pain (DPNP) and Phylopen range - Market leader brand of<br />
“Flucloxacillin” <strong>PC</strong>P also obtained the contract of supplying Dialysis equipment and<br />
consumables to Lanka Hospitals Ltd. In order to facilitate the increasing demand for <strong>PC</strong>P<br />
products the warehouse facilities were also expanded during the year.<br />
2010<br />
As the company’s registered suppliers reached 20, an Enterprise Resource Planning (ERP)<br />
systems was introduced to integrate internal and external management information and 5S<br />
Quality Management System was also introduced to improve the efficiency and<br />
effectiveness of the company.<br />
2011<br />
<strong>PC</strong>P entered in to the FMCG market with the launch of “Celia” nutritional range from<br />
France. The company revenue reached Rs. 500 million during the year. <strong>PC</strong>P was also listed<br />
among the 15 largest pharma importers in the Island.<br />
Product Portfolio<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>s product range consists of the following categories of products,<br />
<strong>Pharma</strong>ceuticals - more commonly known as medicine and drugs – are a fundamental<br />
component of both modern and traditional medicine. It is essential that such products are<br />
safe, effective and of good quality and are prescribed and used rationally. <strong>Pharma</strong>ceuticals<br />
can be further classified in to two categories namely generic pharmaceuticals and branded<br />
pharmaceuticals. Generic pharmaceuticals are copies of brand-name pharmaceuticals that<br />
have exactly the same dosage, intended use, effects, side effects, route of administration,<br />
risks, safety, and strength as the original drug. In other words, their pharmacological effects<br />
are exactly the same as those of their brand-name counterparts.<br />
Nutraceuticals – supplements such as vitamins, minerals, herbs, meal supplements, sports<br />
nutrition products, natural food supplements are used for many purposes. They can be<br />
added to the diet to boost overall health and energy to provide immune system support<br />
and reduce the risk of illness and age related conditions.<br />
Medical Devices – medical devices are used for the purpose of diagnosis, therapy or<br />
surgery. Medical devices include a wide range of products varying in complexity and<br />
applications ex: medical thermometers, blood sugar meter, x-ray machines, dialisers etc.<br />
Medical Consumables – medical consumables such as gloves, couch roll, plasters, cotton<br />
wool, bandages, disposable single use products, gynecology consumables and a wide range<br />
of products are used daily by doctors, nurses as well as in the households.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 17
4.5<br />
The company is also involved in the import and marketing of a reputed brand of Energy<br />
drinks which can be categorized as an FMCG<br />
The <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> product portfolio includes a wide range of western and nonwestern<br />
products and formulations sourced through reputed suppliers across the globe.<br />
<strong>PC</strong>P also offers a wide range of internationally reputed medicines and medical devices like<br />
Glucose Monitoring Systems, Auto sutures, Medical Disposables, Medical Homecare<br />
Apparetus and rehabilitation supports. <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> also imports anti-cancer<br />
pharmaceuticals where suppliers to the local market are very few. Other main categories of<br />
products include anti-diabetic, anti-biotics, ENT and Cardiac preparations, Hormones, and<br />
Biological products sourced from world-class pharmaceutical manufacturers and suppliers.<br />
<strong>PC</strong>P imports, markets and distributes a wide range of products covering the following:<br />
� Gastro-intestinal<br />
� Cardiovascular<br />
� Respiratory<br />
� Central Nervous System<br />
� Anti-Infective<br />
� Endocrine<br />
� Obstetric, Gynaecological, and Urinary-tract disorders<br />
� Malignant disease and immunosuppressant<br />
� Nutrition and Blood<br />
� Musculoskeletal and Joint diseases<br />
� Eye<br />
� ENT products<br />
� Skin<br />
� Anesthetics<br />
� Renal Care Range (Dialysis products)<br />
� Analgesic, Antipyretic, Ant rheumatic and Antispasmodic<br />
� Vitamins and Minerals<br />
� Antalgic and Antihistamine<br />
� Osteoporosis therapy<br />
� Prostatic Disease Products<br />
� Topical Preparations (Antibiotic, Antifungal)<br />
� Antiseptic and Disinfectant<br />
Suppliers<br />
The Company is currently associated with more than 35 reputed suppliers of healthcare<br />
and allied products including the following.<br />
18 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Suppliers of <strong>Pharma</strong>ceutical Products<br />
� Square <strong>Pharma</strong>ceuticals Ltd - Bangladesh,<br />
� T-Man <strong>Pharma</strong>ceuticals Partnership Pvt Ltd., - Thailand,<br />
� Pt. Novell <strong>Pharma</strong>ceutical Laboratories – Indonesia,<br />
� Akums Drugs and <strong>Pharma</strong>ceuticals – India,<br />
� Ethypharm LL – India,<br />
� Apurva Biopharma – India,<br />
� Aurochem <strong>Pharma</strong>ceuticals (Pvt) Ltd,<br />
� Eurolife – India<br />
� IKO Overseas – India,<br />
� Alina Combine – Pakistan<br />
Suppliers of Nutritional Products<br />
� Lactalis International – France<br />
Suppliers of Medical Device<br />
� Asia Connection Co. Ltd – Taiwan,<br />
� Tyson Bioresearch Inc - Taiwan<br />
� Bbraun – Germany,<br />
� Bremed Ltd – Korea,<br />
� Ultramed – Egypt,<br />
� Advance Technology – China,<br />
� Rheamed – Taiwan,<br />
� Greatcare – China,<br />
� M.O.W – Korea,<br />
� Bioland – Korea,<br />
� Farmasino - China<br />
� Folee – China<br />
� Surgimax – Pakistan<br />
Suppliers of Medical Consumables<br />
� Bbraun – Germany<br />
� Farmasino – China<br />
� Ultramed - Egypt<br />
Supplier of FMCG (Energy drink)<br />
� J & D Trading – Korea<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 19
4.6<br />
Distribution<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> adopts the following methods of distribution for their products,<br />
Government Tenders<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> supplies to the government through the government tenders that are<br />
obtained by the company through the Ministry of Health. The company is a leading supplier<br />
of anti-cancer products with over 50% of the tenders floated for anti-cancer products being<br />
awarded to <strong>PC</strong>P due to the very competitive prices offered. It is <strong>PC</strong>P’s policy to maintain<br />
“nominal” margins for life saving pharmaceuticals such as anti-cancer medication such that<br />
all Sri Lankan people could afford these otherwise expensive pharmaceuticals prescribed<br />
for terminal illnesses.<br />
Distribution<br />
Other than supplying to the government the company also sells products through their<br />
island wide distributor network who act as agents to local pharmacies a significant<br />
proportion of pharmaceuticals and basic medical equipment are sold through this method.<br />
Table 4.1: Distributor Network<br />
Name District<br />
Nega Distributors <strong>Colombo</strong><br />
Senitha Agencies Galle<br />
Sandiya Phamaceuticals Galle<br />
Mawlana <strong>Pharma</strong>cy Ampara<br />
Bio <strong>Pharma</strong>cy Batticaloa<br />
Vision <strong>Pharma</strong>cy & Super Market Batticaloa<br />
Vanika Medical Jaffna<br />
New <strong>Pharma</strong>cy & Company Kurunegala<br />
City Medicals Kegalle<br />
Galle Drugs Distributors Kandy<br />
Nancy Distributors Gampaha<br />
Kandana Foods & drugs (Pvt) <strong>Limited</strong> Gampaha<br />
Marketing and Distribution<br />
Nutraceuticals product range, ‘Celia’ is directly marketed by <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>. The range<br />
consists of powdered baby milk and infant cereals which provide everyday nutrition with<br />
three different ranges of products namely basic range, intermediate range and premium<br />
range. The brand also produces medical nutrition for various pathological conditions of<br />
babies as well as formula designed for mothers and expectant mothers.<br />
20 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> also directly markets registered pharmaceuticals to doctors and<br />
hospitals through their sales representatives.<br />
Other<br />
� The Lanka Hospital Corporation PLC<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is in agreement with Lanka Hospital for the exclusive supply of BBraun<br />
dialysis consumables for a period of 5 years ending 2016. According to this agreement , <strong>PC</strong><br />
<strong>Pharma</strong> <strong>Limited</strong> has been instrumental in installing on FOC terms 10 BBraun dialog Plus<br />
dialysis machine at lanka hospital and all dialysis consumables for the dialysis sessions<br />
performed at lanka hospital are purchased exclusively and periodically from <strong>PC</strong> <strong>Pharma</strong><br />
<strong>Limited</strong>.<br />
� Ez Smart blood glucose monitoring systems to S<strong>PC</strong><br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> was awarded the tender for the exclusive supplier of the Ez smart blood<br />
glucose monitoring system (Blood glucose monitoring meters and strips) for the entire<br />
government sector through the S<strong>PC</strong>, <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> provides after sales service for this<br />
product throughout the island by a dedicated experienced team of service specialists on 24<br />
hour on call.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 21
4.7<br />
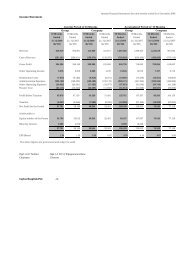
Financial Highlights<br />
Salient extracts from the Income Statement and the Balance Sheet highlighting the<br />
historical performance of the Company during the three year period ended March 31, 2011<br />
and the six months period ended 30 th September 2011 are given below.<br />
Table 4.1 : Financial Performance<br />
Year ended March 31<br />
2009 2010 2011<br />
Audited Audited Audited<br />
Six Months<br />
ended<br />
September<br />
30, 2011<br />
Un -<br />
Audited<br />
Sales 222,432,738 320,036,974 506,902,395 323,854,254<br />
Cost of Sales 146,849,287 206,352,947 216,185,352 137,522,653<br />
Operating Profit 75,583,451 113,684,027 290,717,043 186,331,601<br />
Less: Expenses and Outgoings<br />
Administration and Establishment 31,590,636 38,152,311 54,510,980 31,087,680<br />
Selling and Distribution 28,424,030 34,093,505 50,163,533 28,780,762<br />
Profit from Operations 15,568,785 41,438,211 186,042,530 126,463,159<br />
Less : Finance Cost 11,686,169 17,628,329 18,258,703 11,771,816<br />
Profit before Other Income 3,882,615 23,809,882 167,783,827 114,691,343<br />
Add : Other Income 1,165,164 583,067 191,441 167,721<br />
Net Profit Before Tax 5,047,779 24,392,949 167,975,268 114,859,064<br />
Tax 2,770,033 9,290,604 64,614,863 32,160,538<br />
Net Profit 2,277,746 15,102,345 103,360,405 82,698,526<br />
(Figures are in Rs.)<br />
22 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Table 4.2 : Financial Position<br />
As at March 31 As at<br />
2009 2010 2011 30-Sep-11<br />
Audited Audited Audited Un - Audited<br />
Non Current Assets 8,713,010 6,527,604 5,948,902 8,584,498<br />
Current Assets<br />
<strong>Stock</strong>s 70,984,763 86,998,219 155,676,437 199,543,013<br />
Deposits and Prepayments 595,000 595,000 669,000 1,991,500<br />
Receivables 57,818,619 94,058,364 176,255,858 255,719,244<br />
Treasury Bill - - 5,000,000 5,000,000<br />
Cash and Cash Equivalents 1,340,001 2,282,744 7,726,549 29,727,553<br />
130,738,383 183,934,327 345,327,844 491,981,309<br />
Total Assets 139,451,393 190,461,931 351,276,746 500,565,808<br />
Current Liabilities<br />
Trade and Other Payables 37,891,651 15,915,528 1,018,104 15,653,268<br />
Borrowings 66,474,926 122,466,574 127,731,111 143,127,691<br />
Income Tax Payable 1,135,594 8,380,868 63,076,798 91,127,901<br />
Differed tax liability - - 741,405 741,405<br />
Bank Overdrafts 6,609,577 2,982,223 14,346,296 20,006,580<br />
Non Current Liabilities 2,452,465 727,214 1,013,100 3,860,505<br />
Total Liabilities 114,564,213 150,472,406 207,926,815 274,517,350<br />
Net Assets 24,887,180 39,989,524 143,349,931 226,048,456<br />
Share Capital 21,000,020 21,000,020 101,000,020 101,000,020<br />
Reserves 3,887,160 18,989,505 42,349,911 125,048,436<br />
Total Shareholders' Funds 24,887,180 39,989,525 143,349,931 226,048,456<br />
(Figures are in Rs.)<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 23
4.8<br />
Key Financial Ratios<br />
The key financial ratios of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> for the Financial Years 2008/09, 2009/10 and<br />
20010/11 and for the six months of the Financial Year 2011/12 are illustrated below.<br />
Table 4.3: Key Financial Ratios<br />
Profitability Ratios<br />
Year ended March 31<br />
Six<br />
Months<br />
ended<br />
2009 2010 2011 30-Sep-11<br />
Audited Audited Audited<br />
Un -<br />
Audited<br />
Gross Profit Ratio 33.98% 35.52% 57.35% 57.54%<br />
EBITDA/ Turnover 7.96% 13.43% 37.05% 19.51%<br />
Net Profit Ratio 1.02% 4.72% 20.39% 25.54%<br />
Return on Average Equity 16.84% 46.56% 112.75% 44.75%<br />
Return on Average Total Assets 2.63% 9.16% 38.16% 19.42%<br />
Liquidity Ratios<br />
Debt to Equity Ratio (Times) 2.03 1.29 0.59 0.52<br />
Current Ratio (Times) 1.17 1.23 1.67 1.82<br />
Equity to Asset Ratio (Times) 0.18 0.21 0.41 0.45<br />
Earnings Per Share (Rs.) 1.08 0.15 1.02 0.82<br />
Net Assets Per Share (Rs.) 11.85 0.40 1.42 2.24<br />
� Earnings per Share for the financial years end 31 st March 2010, 31 st March 2011 are<br />
calculated based on the number of shares as at 17 th August 2011 which considers<br />
the bonus share issue of 8,000,000 ordinary voting shares on 1 st October 2010 as<br />
well as the share-split on 17 th August 2011.<br />
24 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
4.9<br />
4.10<br />
Loans, Overdrafts and Other Borrowings<br />
The details of the loans, overdrafts and other borrowings of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as<br />
at November 14, 2011 are depicted below.<br />
Table 4.4: Borrowings of <strong>PC</strong>P as at November 14, 2011<br />
Lender Type of Borrowing<br />
National Development<br />
Bank<br />
National Development<br />
Bank<br />
Short Term<br />
Borrowing<br />
Outstanding<br />
Balance as at<br />
November 14,<br />
2011<br />
Payable<br />
Within one<br />
Year<br />
Payable After<br />
one Year<br />
15,288,535.00 15,288,535.00 -<br />
Import Loans 87,814,411.12 87,814,411.12 -<br />
Commercial Bank Import Loans 8,116,803.90 8,116,803.90 -<br />
National Development<br />
Bank<br />
National Development<br />
Bank<br />
DFCC Bank<br />
National Development<br />
Bank<br />
(Figures are in Rs.)<br />
Imports collection<br />
outstanding<br />
LC acceptance<br />
outstanding<br />
Finance Lease<br />
Liabilities<br />
12,548,485.21 12,548,485.21 -<br />
1,013,528.25 1,013,528.25 -<br />
3,334,606.00 577,994.00 2,756,612.00<br />
Bank Over Draft 13,280,196.66 13,280,196.66 -<br />
There are no guarantees, contingent liabilities and mortgages and charges on the<br />
assets of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as at the date of the Introductory Document.<br />
Dividend Policy and Payments during the Past Three Years<br />
Subject to the provisions of the Companies Act and the Articles of Association of <strong>PC</strong> <strong>Pharma</strong><br />
<strong>Limited</strong>, the declaration and payment of dividends on ordinary voting shares are<br />
recommended and approved by the Board of Directors of the Company. The dividend<br />
policy will be based on a number of factors, including but not limited to the company’s<br />
earnings, capital requirements and overall financial conditions.<br />
Table 4.5 : Dividend Payments<br />
Year ended March 31<br />
2008 2009 2010 2011<br />
Dividends Paid 500,000 550,000 - -<br />
(Figures are in Rs.)<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 25
4.11<br />
4.12<br />
IT Infrastructure<br />
The persistent focus on utilizing information technology to enhance <strong>PC</strong>P’s efficiency and<br />
effectiveness continues to be a main reason behind its success. The function descriptions<br />
below provide an outline of the systems and procedures which have been improved due to<br />
<strong>PC</strong>P’s IT strategy.<br />
� ERP system to ensure better integration of internal and external management<br />
information across the entire organization<br />
� FIFO inventory management system ensures that the goods does not stay in store<br />
for long and better quality products are supplied to the customers<br />
� Optimal working capital management ensuring timely inventory management,<br />
debtor collections and creditors payments<br />
� Financial reporting system enabling the delivery of accurate and relevant<br />
information to management<br />
� Human resources management<br />
Accreditation and Certifications<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> continues to improve their high standards of service with the aim of<br />
supplying better quality products to customers.<br />
� ISO standards, <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is the first Sri Lankan pharmaceutical importer<br />
to obtain ISO 9001:2000, an award signifying the implementation of vital standards<br />
in the organization while streamlining systems and processes. In 2011 <strong>PC</strong>P<br />
obtained the recertification ISO standards ISO 9001:2008 certification which is<br />
awarded to companies that meet ISO requirements in systems & processes,<br />
management, resources, control mechanisms and remedial activities was also<br />
awarded to <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>.<br />
� All products imported and distributed by <strong>PC</strong>P are either GMP or WHO certified.<br />
GMP stands for Good Manufacturing Practices which is required to be adopted by<br />
pharmaceutical manufacturing and testing companies. WHO certification ensures<br />
that the products are of highest quality.<br />
26 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
5.<br />
5.1<br />
Future Strategies and Risk Analysis<br />
Future Strategies<br />
The company has an optimistic view of the <strong>Pharma</strong>ceuticals Industry in Sri Lanka and is<br />
focused on achieving double digit growth in its turnover and profits during the foreseeable<br />
future. The company’s future plans include the following.<br />
Acquisitions and Strategic Alliances with Healthcare Suppliers and Manufacturers<br />
Having identified the intensifying competition in the pharmaceutical sector, the company<br />
plans to enter into strategic alliances with key suppliers and manufacturers to increase<br />
profitability as well as to improve the range of products. Company will also actively seek<br />
opportunities for acquisition of entities which will bring in strategic benefits. The company<br />
is in the process of negotiating with several Healthcare suppliers and manufacturers for<br />
strategic alliances and acquisitions.<br />
Diversification<br />
The demands for nutraceuticals are expected to increase due to an aging population and a<br />
rise in income levels which in turn increases the focus on health and wellness. The<br />
company also expects to expand its presence in the energy drinks market which promises<br />
high growth potential.<br />
To Setup a <strong>Pharma</strong>ceutical Manufacturing Operation<br />
The company intends to move into the production of pharmaceutical products by during<br />
the next three years with the rapid increase in demand for such products, having a<br />
manufacturing plant would allow <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> to supply pharmaceuticals at a<br />
relatively lower cost and hence boost its profitability. The company has submitted the<br />
expression of interest on 3 rd November 2011 and now is in negotiations with the<br />
government of Sri Lanka for the establishment of a pharmaceutical manufacturing plant in<br />
Kurunegala.<br />
Establishment of a Distribution Network<br />
The company intends to establish its very own fleet of delivery vehicles which would allow<br />
them to deliver products Island wide more efficiently and effectively. <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
will also establish an emergency delivery service for ‘life saving pharmaceuticals’ which<br />
would allow customers access to pharmaceuticals at times of need.<br />
Continuous Efforts in Improving Efficiency and Productivity<br />
The company will continue to enhance its efficiency and the productivity on supply<br />
management and distribution by improving internal processes and investing in more<br />
effective internal control systems.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 27
5.2<br />
5.3<br />
Underlying Assumptions on Which Future Strategies Are Based on<br />
It is conceited that the business of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> will continue to expand at or above<br />
the historical average in near future in line with the rapid development in the healthcare<br />
sector.<br />
In addition the Company expects that the demand and expectations of its selected clients<br />
will not vary to an unprecedented degree during the next few years and the company will<br />
prolong its partnership with its existing clientele to retain the current business<br />
opportunities and to achieve in new prospects.<br />
The company expects to fund the expansions through internally generated funds and<br />
suitable bank borrowings when necessary.<br />
Market Approach<br />
Quality First<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is the first Sri Lankan <strong>Pharma</strong>ceutical importer to obtain ISO 9001:2008,<br />
an award signifying the implementation of vital standards in the organization while<br />
streamlining systems and processes. ISO 9001:2008 certification is a testimony to <strong>PC</strong>P’s<br />
commitment to companies meet ISO requirements in systems & processes, management,<br />
resources, control mechanisms, and remedial activities.<br />
People First<br />
Quality is delivered through people and the <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> team is dedicated, welltrained<br />
and experienced and stringently follows the Company’s quality standards and code<br />
of ethics. At <strong>PC</strong>P, promoting a health-conscious lifestyle is every employee’s responsibility.<br />
The Company takes pride in employing experienced and professionally qualified marketers<br />
and support personnel to represent, design customer value and creates strong brand<br />
equity. Well-trained, dedicated and empowered promotional field teams market the<br />
Company’s products throughout the island, backed by talented and enthusiastic in-house<br />
support services personnel.<br />
Our frontline staff is taught to listen to customers. A predominantly market oriented<br />
culture ensures that customer satisfaction remains the focus of the organization’s<br />
offerings. Due to the strong customer orientation, relationship building skills and<br />
networking capabilities we have developed a good rapport with doctors, pharmacists and<br />
end-consumers.<br />
28 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
5.4<br />
Associated Risks Related to Future Strategies<br />
Several factors could affect the success of the future strategies,<br />
Macro Economy<br />
The company’s future plans are based on an expectation of rapid economic growth in Sri<br />
Lanka over the foreseeable future. Changes to macro-economic factors including<br />
disposable income levels and market interest rates will have a direct bearing on the<br />
feasibility of new ventures, and will affect the company’s future plans for expansion and<br />
diversification.<br />
Government Policies<br />
As a stringently regulated industry with national and political implication pharmaceuticals<br />
industry is vulnerable to changes in government policy towards healthcare and<br />
pharmaceuticals. Further certain strategies may be obstructed due to delays in the<br />
approval process of the Health Ministry or the Drug Regulatory Authority.<br />
Foreign Currency Risk<br />
Since <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is primarily an import oriented entity the costs are incurred in<br />
foreign currency. Moreover the suppliers are also subject to various regulations and legal<br />
requirements by the governments as well as from WHO. The depreciation of Sri Lankan<br />
Rupee against US dollars tends to increase the costs and reduce profitability of the<br />
company creating a negative impact for the company.<br />
People Risk<br />
The experienced key management team together with the skilled workforce has played a<br />
vital role in achieving today’s success of the company. In an environment where it has been<br />
a challenge to attract and retain right people with right talent, significant change in the<br />
management structure or trained workforce could cause interruption to service standards<br />
which may have an adverse impact on company’s performance.<br />
Risk of Reliance on External Suppliers<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> relies on external suppliers for pharmaceuticals and equipment, any<br />
delays or non-conformance to quality requirements caused by our suppliers may result in<br />
disruption in operations, unexpected cost escalation and customer complaints and even<br />
government action which will have an impact on company’s immediate and long term<br />
profitability.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 29
Risk associated with the Manufacturing Process<br />
Given the nature of the products there are several manufacturing related risks can be<br />
identified,<br />
The manufacturing of pharmaceuticals is subject to a very strict process which consists of<br />
strict regulations on the quality of raw material supplied, quality control through the<br />
formulation process, and packaging of the finished products as well as the distribution<br />
process. The process and standards will vary according to the product hence careful<br />
attention should be paid to each product at each stage any mishap in the process would<br />
result in a low quality products this will be harmful to the patient and have an adverse<br />
impact on the company.<br />
Scrap disposal will be one of the biggest issues in maintaining a pharmaceutical<br />
manufacturing plant as each item of waste will have to be disposed in such a way so that it<br />
won’t be harmful to the environment or the people hence the process of disposing will be<br />
costly. Another potential risk is the risk of recalls, if a product is not up to the required<br />
quality standards the company will have to recall the products from the distributors and<br />
this will have a severe impact on the company’s financials.<br />
Given the nature of production there will be strict regulations and quality tests imposed by<br />
the government in order to ensure the quality of the products, the company will be<br />
required to obtain licenses from the required authorities and if the company fails to<br />
maintain the quality standards then they will be exposed to the risk of losing the license.<br />
Any of the said risks would have a negative impact on the company’s immediate and longterm<br />
profitability.<br />
30 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
6.<br />
6.1<br />
Human Resources<br />
Staff Strength<br />
The staff strength of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as at 14 th November, 2011 was 82 employees. The<br />
composition of the staff base of the company is detailed in the Table 6.1.<br />
Table 6.1 : Staff composition of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong><br />
Rank<br />
No of<br />
employees<br />
Corporate Management level 8<br />
Managerial Level 8<br />
Executive Level 56<br />
Clerical level 10<br />
Total 82<br />
The Company’s employees have not arrived at any collective agreements and are not<br />
unionised.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 31
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 32
6.2<br />
Corporate Management Team<br />
Dilshad Mohamed Ikram – General Manager<br />
Dilshad started his career in 1993 as a Trainee Medical Representative at a pioneering<br />
pharmaceutical organization. He holds over 15 years management experience in the<br />
pharmaceutical industry and has been instrumental in the launch and growth of many<br />
successful medicines locally. He has management experience of many international<br />
agencies and became the first to launch a Bangladeshi pharmaceutical agency locally in<br />
1998. Dilshard has been a founder member of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> and has spearheaded the<br />
organization from its grassroots since its inception.<br />
A.A.D.T.P. Saparamadu – Senior Marketing Manager<br />
Saparamadu brings over 21 years of experience in the <strong>Pharma</strong>ceutical industry. Over the<br />
years he has held many managerial posts in Sales and Marketing at Multinational<br />
<strong>Pharma</strong>ceutical Organizations locally.<br />
A.S.W. Vithana - National Sales Manager<br />
Vithana holds an MBA and Diploma in Marketing and Diploma in Sales from University of<br />
Manipal. He holds over 14 years of experience in the industry and was an integral part in<br />
the foundation of the State <strong>Pharma</strong>ceutical Corporation (SPMC) and in the launching of its<br />
local manufacturing of pharmaceuticals.<br />
G.A.R.M. Shifa – Marketing Manager<br />
Shifa brings in over 15 years of experience in pharmaceuticals including international<br />
experience to the wealth of experience <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> already possesses. He is<br />
presently reading the final stage of the MBA from University of Wales Institute of Cardiff.<br />
Over his career he has been awarded many awards for sales performance locally and<br />
internationally.<br />
Samitha Danwatte – Marketing Manager – Nutritional<br />
Samitha holds an MBA from Buckinghamshire University and brings over 18 years of<br />
experience in the <strong>Pharma</strong>ceutical Industry as a Marketing Executive, Business Manager,<br />
Sales & Marketing Manager as well as a Marketing Manager at several reputed<br />
organizations.<br />
Mr. Danwatte also holds a Diploma in Marketing and a Post Graduate Diploma in<br />
Management awarded by Sri Lanka Institute of Marketing (SLIM) as well as a Diploma in<br />
Finance Management from Wigan & Leigh College – UK.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 33
Natasha Antoinette Abeyagoonasekera - Manager International Business<br />
Natasha has been at <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> since the inception and coordinates with<br />
International Agencies held by the company. She brings over 10 years’ experience in the<br />
industry and is a Graduate in Marketing awarded by the Chartered Institute of Marketing<br />
(CIM) – UK. She also holds a Degree in Business Administration awarded by the Association<br />
of Business Executives (ABE) – UK. Natasha is a Member of the Chartered Management<br />
Institute (MCMI) – UK and has been the World Prize Winner in “Strategic Marketing<br />
Management” (Ray Thomas prize) of the same institute. Further she holds a Diploma in<br />
<strong>Pharma</strong>ceutical Studies.<br />
J. D. Lakshman Dinuka – Business Development Manager<br />
Dinuka has been the Business Development Manager of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> since 2009 and<br />
brings over 12 years of experience in the <strong>Pharma</strong>ceutical Industry including 3 years of<br />
experience as a Field Manager.<br />
Mahesh Kumarashinghe – Finance Manager<br />
Mahesh is an Associate member of the Institute of Chartered Accountants of Sri Lanka. He<br />
has over 13 years of experience in providing Accounting & Finance services in various<br />
industries including Manufacturing, Export, Automotive, Construction and Healthcare with<br />
local & foreign exposure. He holds a Higher National Diploma in Accountancy from Sri<br />
Lanka Institute of Advanced Technological Education and is an Associate Member of the<br />
Society of Certified Management Accountants of Sri Lanka.<br />
There are no management agreements presently in force or currently being considered by<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>.<br />
34 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
7.<br />
7.1<br />
Corporate Structure<br />
Share Capital Structure<br />
As at 14th November 2011 the stated capital of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> is Rs. 101,000,020<br />
comprising of 101,000,020 issued and fully paid ordinary voting shares.<br />
As at 30 th September 2010, the stated capital of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> was Rs. 21,000,020<br />
comprising of 2,100,002 issued and fully paid ordinary voting shares. On 1st October 2010<br />
the company transferred 2,100,002 shares to <strong>PC</strong>H Holdings <strong>Limited</strong> which was followed by<br />
a bonus share issue of 8,000,000 ordinary voting shares in the name of <strong>PC</strong>H Holdings<br />
<strong>Limited</strong> on the same day.<br />
Subdivision of shares<br />
On 17th August 2011 it was resolved by means of a special resolution that each of the<br />
issued and subscribed 10,100,002 Ordinary Shares were subdivided in to 10 fully paid<br />
Ordinary Shares increasing the number of Ordinary Voting Shares of the company to<br />
101,000,020.<br />
Shares redeemed or repurchased<br />
The company has not been engaged in any share re-purchase, redemption or stated capital<br />
reduction exercises in the two years preceding the date of this Introductory Document.<br />
Outstanding convertible Debt Securities<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> has no outstanding convertible Debt Securities.<br />
Foreign holding restrictions<br />
There are no restrictions on the purchase of shares of the company by non-residents.<br />
Free transferability of securities<br />
There are no statutory restrictions on the free transferability of the issued shares.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 35
7.2<br />
Share Capital<br />
Share Allotments in the Preceding Two Years<br />
<strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> has not issued any shares during the last two years preceding the date<br />
of this Introductory Document in addition to the share issues tabulated in the Table 7.1<br />
below.<br />
Table 7.1 : Share Capital Details<br />
Date of Allotment Type No of shares Share Price<br />
Share Capital<br />
(Rs.)<br />
As at September 30 , 2009 2,100,002 10.00 21,000,020<br />
October 1, 2010 Bonus Issue 8,000,000 10.00 80,000,000<br />
As at March 31, 2011 10,100,002 10.00 101,000,020<br />
August 17, 2011 Share Split 101,000,020 1.00 101,000,020<br />
As at November 14, 2011 101,000,020 101,000,020<br />
Transfer of Shares – November 5, 2011<br />
13,510,000 ordinary shares of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>, which were held by <strong>PC</strong>H Holdings<br />
<strong>Limited</strong>, were transferred to the following shareholders at a price of Rs. 10.00 per share on<br />
5 th November, 2011.<br />
Table 7.2 – Transfer of Shares<br />
Shareholder<br />
No of shares<br />
held before<br />
the transfer<br />
No of shares<br />
transferred<br />
No of shares<br />
held after<br />
transfer<br />
<strong>PC</strong>H Holdings <strong>Limited</strong> 101,000,020 (13,510,000) 87,490,020<br />
Mr. M.R .M. Khalideen - 4,000,000 4,000,000<br />
Mr. M.R .M. Nawaz - 5,000,000 4,000,0005,00 87,490,020 5,000,000<br />
Mr. V.G.A.C. Mohamed Mubarak - 2,500,000 5,000,000 2,500,000<br />
Thambapanni Investments <strong>Limited</strong> - 2,000,000 2,500,000 2,000,000<br />
Others - 10,000 2,000,000 10,000<br />
10,000<br />
Other than the share transfer which took place on 5 th November 2011, no other shares of<br />
the company have been transferred or sold by the major shareholder within the immediate<br />
period of one year from the date of this Introductory Document.<br />
36 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
7.3<br />
Shareholding Structure<br />
Major Shareholders<br />
Tabulated below is the list of major shareholders of <strong>PC</strong>P as at 14 th November, 2011<br />
Table 7.3 – Major Shareholders of <strong>PC</strong>P as at 14 th November, 2011<br />
Shareholder No of shares % of issued capital<br />
<strong>PC</strong>H Holdings <strong>Limited</strong> 87,490,020 86.62%<br />
Mr. M.R. Mohamed Khalideen 4,000,000 3.96%<br />
Mr. M.R. Mohamed Nawaz 5,000,000 4.95%<br />
Mr. V.G.A.C. Mohamed Mubarak 2,500,000 2.48%<br />
Thambapanni Investments <strong>Limited</strong> 2,000,000 1.98%<br />
Others 10,000 0.01%<br />
Total 101,000,020 100.00%<br />
Others comprise of 100 individual shareholders with each person holding 100 shares.<br />
Public Holding<br />
Public holding of <strong>PC</strong>P shares as at 14 th November, 2011 is 13,510,000 shares which is<br />
13.38% of the total and is held by 104 public shareholders.<br />
Tabulated below is the list of public holding among 104 shareholders of <strong>PC</strong>P as at 14 th<br />
November, 2011<br />
Table 7.4 – Public Holding of <strong>PC</strong>P as at 14 th November, 2011<br />
Shareholder No of shares % of issued capital<br />
Mr. M.R. Mohamed Khalideen 4,000,000 3.96%<br />
Mr. M.R. Mohamed Nawaz 5,000,000 4.95%<br />
Mr. V.G.A.C. Mohamed Mubarak 2,500,000 2.48%<br />
Thambapanni Investments <strong>Limited</strong> 2,000,000 1.98%<br />
Others* 10,000 0.01%<br />
Total 13,510,000 13.38%<br />
*Others comprise of 100 individual shareholders with each person holding 100 shares.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 37
Distribution of Shareholding<br />
Distribution of shareholding of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> shares as at 14 th November, 2011 is<br />
depicted in the Table 7.5.<br />
Table 7.5 : Distribution of shareholding as at 14 th November, 2011<br />
From To<br />
No of<br />
shareholders<br />
No of shares %<br />
10 - 10,000 100 10,000 0.01<br />
10,001 - 100,000 0 0 0.00<br />
100,001 - 1,000,000 0 0 0.00<br />
1,000,001 - 10,000,000 4 13,500,000 13.37<br />
Over 10,000,000 1 87,490,020 86.62<br />
Total 105 101,000,020 100.00<br />
Analysis of Shareholders<br />
Analysis of shareholders based on the type of shareholder as at 14 th November, 2011 is<br />
illustrated in the Table 7.6.<br />
Table 7.6 : Analysis of Shareholders as at 14 th November, 2011<br />
No of shareholders<br />
No of shares %<br />
Individual 103 11,510,000 11.4<br />
Institutions 02 89,490,020 88.6<br />
Total 105 101,000,020 100.0<br />
38 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
8.<br />
Board of Directors<br />
As at the date of this Introductory Document, the Board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> comprises<br />
the following six Directors.<br />
Name Residential Address Other Directorships<br />
Mr. S.H.M Rishan<br />
Mrs. S. Rishan<br />
Mr. Mushtaq Ahmed<br />
Ikram<br />
Mr. Ramasamy Selvaraj<br />
Mr. Prasanna Lakshantha<br />
Wijesekara<br />
No - 01<br />
Charles Circus<br />
<strong>Colombo</strong> 03<br />
No - 01<br />
Charles Circus<br />
<strong>Colombo</strong> 03<br />
No-49<br />
Quarry Road<br />
Dehiwala<br />
No – 608 – 3/9<br />
Baseline Road<br />
<strong>Colombo</strong> 9<br />
No- 344<br />
High Level Road<br />
Mahalwarawa<br />
Pannipitiya<br />
� <strong>PC</strong> House PLC<br />
� Orients Garments PLC<br />
� <strong>PC</strong>H Holdings <strong>Limited</strong><br />
� Greenwich Lanka (Pvt)<br />
Ltd<br />
� Sansiyo Corporation<br />
(Pvt) Ltd<br />
� Procifinity <strong>Limited</strong><br />
� Info Serve (Pvt) Ltd<br />
� <strong>PC</strong> House PLC<br />
� <strong>PC</strong>H Holdings <strong>Limited</strong><br />
� Sansiyo Corporation<br />
(Pvt) Ltd<br />
� <strong>PC</strong> House PLC<br />
(Alternate Director to<br />
Mrs Shithy Sharmila<br />
Rishan)<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 39<br />
N/A<br />
N/A
8.1<br />
Mrs. Shanthi Sri Nanda<br />
Goonaratne<br />
Profile of Directors<br />
No- 7/3<br />
Kottage Watte Road<br />
Sri Subuthipura<br />
Battaramulla<br />
Mr. S.H.M. Rishan - Chairman/ Chief Executive Officer<br />
� Samanala Power (Pvt)<br />
<strong>Limited</strong><br />
Mr. Rishan is the Founder Chairman and Chief Executive Officer of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> and<br />
<strong>PC</strong> House PLC. His visionary leadership enabled <strong>PC</strong> House PLC to grow from its humble<br />
beginnings to be one of the largest ICT companies in Sri Lanka. He completed his secondary<br />
education at Kingswood College, Kandy and commenced his career as a sailor after<br />
graduating from the Moratuwa Marine Academy. Mr. Rishan counts over 14 years of<br />
experience in the ICT industry and over 5 years in <strong>Pharma</strong>ceutical and has been awarded by<br />
many local and foreign bodies for his outstanding entrepreneurship.<br />
Mrs. Sharmila Rishan - Non Executive Director<br />
Mrs. Rishan has been serving on the board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> since its inception. She is<br />
also a director of <strong>PC</strong>H Holdings <strong>Limited</strong> and <strong>PC</strong> House PLC.<br />
Mr. Mushtaq Ahmed Ikram – Executive Director<br />
Mr. Ikram has worked in many leading local and overseas banks including Indian Bank, ABN<br />
Amro, Nation Trust Bank PLC and Dubai Bank holding many senior positions and counts<br />
over 18 years experience in the banking sector. He also served as the finance controller in<br />
several other reputed companies. Mr. Ikram holds a Postgraduate Executive Diploma in<br />
Bank Management from the Finance institute of Bankers of Sri Lanka. Ikram is presently an<br />
Alternate Director at <strong>PC</strong> House PLC.<br />
Mr. Ramasamy Selvaraj – Non-Executive Independent Director<br />
Mr. Selveraj was appointed to the Board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as a Non-Executive<br />
Independent Director in 2011. He is an Ex Senior Banker who started his career from the<br />
grass root levels and rose to very senior level of leading private banks in Sri Lanka. He is an<br />
expert in Risk and Credit Management and Consumer Banking. He also work’s as a resource<br />
person in banking studies attached to the Institute of Bankers. He counts for more than 25<br />
years of banking experience.<br />
Mr. Prasanna Wijesekara – Non-Executive Director<br />
Mr. Prasanna Wijesekara was appointed to the Board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as a Non-<br />
Executive Director. Mr. Prasanna Wijesekara is an Associate Member of the Institute of<br />
Chartered Accountants of Sri Lanka. He counts for over 14 years of experience in the field<br />
of Accountancy and has worked in several reputed Companies in Sri Lanka.<br />
40 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
8.2<br />
8.3<br />
8.4<br />
Mrs. Shanthi Sri Nanda Goonaratne – Non-Executive Independent Director<br />
Mrs. Shanthi was appointed to the Board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> in 2011. She is a top<br />
professional with more than 35 years of experience in Banking, Manufacturing and Power<br />
Generation sectors. Her banking career spans across both Public and Private sectors as well<br />
as in Commercial and Investment Banking and she held the top positions in Credit and<br />
Operations. She also held the top positions of Chief Executive and Chairman of successful<br />
private ventures in Manufacturing and Power Generation. She is a graduate from<br />
University of Peradeniya and earned her MBA from University of Sri Jayawardenepura. She<br />
is also an Associate Member of Institute of Bankers and a Member of the Personnel<br />
Management Institute of Sri Lanka.<br />
Directors’ Interest in Shares<br />
Shares held by the Directors<br />
The Directors’ direct shareholdings in <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> as at the date of this Introductory<br />
Document are tabulated below.<br />
Table 8.1 - Shares Held by the Directors<br />
Name of the Director No. of Shares Held<br />
Mr. M. A. Ikram 100<br />
Statement – Chairman/ Chief Executive Officer<br />
The Chairman/ Chief Executive Officer of the Company has not been involved in any of the<br />
following:<br />
� A petition under any bankruptcy laws filed against such person or any partnership in<br />
which he was a partner or any corporation of which he was an executive officer.<br />
� Conviction for fraud, misappropriation or breach of trust or any other similar offence<br />
which the CSE considers a disqualification.<br />
Statement – Board of Directors<br />
No director or a person nominated to become a director of the Company has been involved<br />
in any of the following:<br />
� A petition under any bankruptcy laws filed against such person or any partnership in<br />
which he was a partner or any corporation of which he was an executive officer.<br />
� Conviction for fraud, misappropriation or breach of trust or any other similar offence<br />
which the CSE considers a disqualification.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 41
8.5<br />
8.6<br />
Director’s Emoluments<br />
Emoluments for directors were not paid for the financial year 2010/2011 however it is<br />
estimated that for the financial year 2011/2012 Director’s emoluments would amount<br />
to approximately Rs. 1.5 Mn.<br />
Other Disclosures<br />
Directors’ Interest in Assets and Contracts<br />
The Directors hold no interests in any assets acquired, disposed or leased by <strong>PC</strong> <strong>Pharma</strong><br />
<strong>Limited</strong> in the past two years preceding the date of this Introductory Document. As at date<br />
27 th December 2011, the Directors have no interest in any assets proposed to be acquired,<br />
disposed or leased by the Company during the next 2 years.<br />
There are no other contracts or arrangements inforce as at the date of this Introductory<br />
Document in which the Directors of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> are materially interested in relation<br />
to the business of the company.<br />
Details of Commission Paid<br />
No commission has been paid in the two years preceding the date of this Introductory<br />
Document for subscribing or agreeing to subscribe or procuring or agreeing to procure<br />
subscriptions for any shares of the Company.<br />
Details of Benefits Paid to Promoters<br />
No benefit has been paid or given to any promoter within the 2 years preceding the date of<br />
this Introductory Document and there are no benefit intended to be paid or given to any<br />
promoter.<br />
Material Contracts<br />
The Company has not entered into any material contracts other than those contracts<br />
entered into in the ordinary course of business.<br />
Rights and Obligations Attached to the Shares<br />
Ordinary voting shares of the Company will have the right to vote, the right to an equal<br />
share in any dividend that may be declared by the Company and the right to an equal share<br />
in the distribution of the surplus assets of the Company in liquidation.<br />
42 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
9.<br />
Other Information Relating to the Company<br />
Degree of Dependence on Key Suppliers<br />
The company is not significantly dependent on any single supplier for pharmaceuticals,<br />
medical consumables and medical devices. Currently the company has a single supplier for<br />
its nutraceuticals and FMCGs lines.<br />
Degree of Dependence on Key Customers<br />
The company derives its business from a wide range of customers ranging from the GOSL,<br />
private healthcare institutions and pharmacies. Purchase decisions of GOSL as the single<br />
largest customer of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> will affect company’s revenue and profit figures.<br />
Litigation, Disputes and Contingent Liabilities<br />
As at the date of this Introductory Document, there are no material-legal arbitration or<br />
mediation proceedings which may have or have had in the recent past affected the<br />
financial position or profitability of the Company.<br />
As at the date of this Introductory Document, there are no contingent liabilities that would<br />
affect current and future profits of the Company.<br />
As at the date of this Introductory Document, there are no penalties imposed by any<br />
regulatory or state authority against the Company.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 43
10.<br />
Corporate Governance Practices<br />
The Board of Directors of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> has given the due emphasis to the importance<br />
of highest standard corporate governance practices in promoting business integrity,<br />
accountability and transparency.<br />
The following framework forms the of Corporate Governance code of the Company.<br />
Constitution of the Board<br />
The Company has two Executive Directors, two Non-Executive Directors and two Non-<br />
Executive Independent Directors on the Board with the expertise mainly in the areas of<br />
financial management, general management and operations of <strong>Pharma</strong>ceutical companies.<br />
Non-Executive Directors<br />
The Board of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> consists of the following Non-Executive Directors.<br />
� Mrs. Sithy Sharmila Rishan<br />
� Mr. Prasanna Lakshantha Wijesekara<br />
� Mr. Ramasamy Selvaraj<br />
� Mrs. Shanthi Sri Nanda Goonaratne<br />
Non-Executive Independent Directors<br />
The following directors of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> have fulfilled the requirements to be Non-<br />
Executive Independent Directors of the Company.<br />
� Mr. Ramasamy Selvaraj<br />
� Mrs. Shanthi Sri Nanda Goonaratne<br />
Remuneration Committee<br />
The Remuneration Committee consists of two Independent Non-Executive Directors and<br />
one Non-Executive Director. Mr. Ramasamy Selvaraj functions as the Chairman of the<br />
Remuneration Committee.<br />
� Mr. Ramasamy Selvaraj – Non Executive Independent Director<br />
� Mrs. Shanthi Sri Nanda Goonaratne – Non Executive Independent Director<br />
� Mr. Prasanna Lakshantha Wijesekara – Non Excutive Director<br />
The Remuneration Committee is responsible for making recommendations to the Board on<br />
the remuneration of Executive Directors and Non-Executive Directors. The committee is<br />
also responsible for setting up the remuneration policy, which provides guidelines to the<br />
Board on the overall remuneration framework to ensure that remuneration levels are at a<br />
satisfactory level to attract and retain the requisite professionals to support the success of<br />
the Company.<br />
44 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Audit Committee<br />
The Audit Committee comprises of the following one Non-Executive Director and two<br />
Independent Non-Executive Directors.<br />
� Mr. Prasanna Lakshantha Wijesekara – Non-Executive Director<br />
� Mr. Ramasamy Selvaraj – Non Executive Independent Director<br />
� Mr. Shanthi Sri Nanda Goonaratne – Non-Executive Independent Director<br />
Mr. Prasanna Lakshantha Wijesekara functions as the Chairman of the Audit Committee.<br />
The Chief Executive Officer and the Finance Manager attends meetings of the Audit<br />
Committee by invitation.<br />
The Audit Committee is responsible for reviewing the functions and processes of internal<br />
controls in the Company and ensuring the effectiveness of such controls. The Committee<br />
monitors all audit activities and operations and ensures their compliance with overall<br />
policies and procedures as well as adherence to statutory and regulatory requirements and<br />
industry best practices.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 45
11.<br />
Statutory Declaration<br />
Declaration by the Board of Directors<br />
Date: 27 th December 2011<br />
We the undersigned, who are named in the Introductory Document as Directors of <strong>PC</strong><br />
<strong>Pharma</strong> <strong>Limited</strong>, hereby declare and confirm that we have read the provisions of the CSE<br />
listing rules and of the Companies Act No. 07 of 2007 and any amendments to it relating to<br />
the issue of this Introductory Document and those provisions have been complied with.<br />
This Introductory Document has been seen and approved by us and we collectively and<br />
individually accept full responsibility for the accuracy of the information given and confirm<br />
that after making all reasonable enquires and to the best of our knowledge and belief,<br />
there are no other facts the omission of which would make any statement herein<br />
misleading or inaccurate.<br />
Where representations regarding the future performance of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> have been<br />
given in the Introductory Document, such representations have been made after due and<br />
careful enquiry of the information available to <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> and making assumptions<br />
that are considered to be reasonable at the present point in time in our best judgment.<br />
Name Designation Signature<br />
Mr. Saheedul Hijiry Mohamed Rishan Chairman (Signed)<br />
Mrs. Sithy Sharmila Rishan Director (Signed)<br />
Mr. Mushtaq Ahmed Ikram Director (Signed)<br />
Mr. Ramasamy Selvaraj Director (Signed)<br />
Mr. Prasanna Lakshantha Wijesekara Director (Signed)<br />
Mrs. Shanthi Sri Nanda Goonaratne Director (Signed)<br />
46 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
12.<br />
Statutory and Other General Information<br />
Inspection of Documents<br />
The Introductory Document and The Articles of Association of <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> will be<br />
available on the website of the CSE (www.cse.lk) and on the Company website<br />
(http://www.pcpharma.lk) for a period of not less than 14 days.<br />
Reports by Experts<br />
Apart from the Independent Auditors Report, this Introductory Document does not<br />
include any other reports or statements made by experts.<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 47
Audited Financial Statements<br />
48 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Annexure I - Auditors Report and Financial Statements as at March 31, 2009<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 49
50 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 51
52 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 53
54 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 55
56 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 57
58 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 59
60 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 61
62 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 63
Annexure II - Auditors Report and Financial Statements as at March 31, 2010<br />
64 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 65
66 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 67
68 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 69
70 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 71
72 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 73
74 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 75
76 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 77
78 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Annexure III - Auditors Report and Financial Statements as at March 31, 2011<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 79
80 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 81
82 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 83
84 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 85
86 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 87
88 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 89
90 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 91
92 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 93
94 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 95
Interim Financial Statements<br />
96 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Annexure IV - Interim Financial Statements as at September 30, 2011<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 97
98 Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong>
Annexure IV - Interim Financial Statements as at September 30, 2011<br />
Introductory Document – <strong>PC</strong> <strong>Pharma</strong> <strong>Limited</strong> 97