IN THIS ISSUE - Drug Development & Delivery
IN THIS ISSUE - Drug Development & Delivery
IN THIS ISSUE - Drug Development & Delivery
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CHOOS<strong>IN</strong>G THE APPROPRIATE<br />
TECHNOLOGY<br />
Bend Research takes an “agnostic”<br />
approach to choosing the appropriate<br />
solubilization technology, guided by a<br />
number of factors, including the<br />
compound’s physical-chemical properties,<br />
the projected dose, the desired release<br />
profile, and the results from numerous<br />
formulation and process models that we<br />
have developed throughout the past<br />
15 years.<br />
The goal in choosing the optimum<br />
solubilization technology is aimed at<br />
solving the right problem for that specific<br />
compound. Bend Research is capitalized<br />
for formulation development and cGMP<br />
manufacturing for spray-drying, hot-melt<br />
extrusion, and particle-size reduction to<br />
support our agnostic approach.<br />
Additionally, we can support the<br />
formulation of self-emulsifying and lipid<br />
formulations relying on a partner to<br />
complete the cGMP manufacturing.<br />
While we consider all possible<br />
technologies as we develop formulation<br />
approaches for poorly soluble compounds,<br />
our experience in the formulation of more<br />
than 500 low-solubility compounds is that<br />
spray-drying amorphous dispersions is the<br />
most widely applicable approach. This<br />
process involves dissolving the compound<br />
and a concentration-sustaining polymer in<br />
a volatile organic solvent that is rapidly<br />
removed in the spray dryer. This process<br />
allows for rapid drying of the formulation<br />
and isolation of the amorphous dispersion.<br />
It is widely applicable in that it avoids<br />
either melting the compound in a hot-melt<br />
process or relying on the solubility of the<br />
compound in a lipid vehicle.<br />
An additional benefit is that the<br />
spray-drying process has been scaled<br />
down to small scales compatible with<br />
early preclinical quantities of compound<br />
and scaled up to the multi-ton scales<br />
necessary for commercial manufacture.<br />
SPRAY-DRIED DISPERSIONS:<br />
KEY FACTORS & PROCESS<br />
OVERVIEW<br />
There are three key components to<br />
obtaining a high-performing amorphous<br />
dispersion. These are the spray-drying<br />
process conditions, the identification of<br />
the compound’s physical-chemical<br />
properties, and finally, the choice of<br />
excipients that are used in the amorphous<br />
dispersion.<br />
The spray-drying process is<br />
conceptually quite simple. Initially, the<br />
compound and a dispersion polymer are<br />
dissolved in a volatile organic solvent<br />
(e.g., acetone or methanol). The solution is<br />
then introduced into the drying chamber<br />
of a spray dryer along with heated<br />
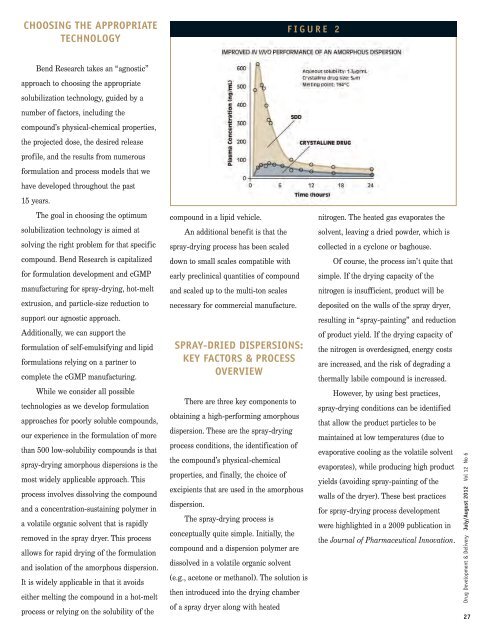
F I G U R E 2<br />
nitrogen. The heated gas evaporates the<br />
solvent, leaving a dried powder, which is<br />
collected in a cyclone or baghouse.<br />
Of course, the process isn’t quite that<br />
simple. If the drying capacity of the<br />
nitrogen is insufficient, product will be<br />
deposited on the walls of the spray dryer,<br />
resulting in “spray-painting” and reduction<br />
of product yield. If the drying capacity of<br />
the nitrogen is overdesigned, energy costs<br />
are increased, and the risk of degrading a<br />
thermally labile compound is increased.<br />
However, by using best practices,<br />
spray-drying conditions can be identified<br />
that allow the product particles to be<br />
maintained at low temperatures (due to<br />
evaporative cooling as the volatile solvent<br />
evaporates), while producing high product<br />
yields (avoiding spray-painting of the<br />
walls of the dryer). These best practices<br />
for spray-drying process development<br />
were highlighted in a 2009 publication in<br />
the Journal of Pharmaceutical Innovation.<br />
<strong>Drug</strong> <strong>Development</strong> & <strong>Delivery</strong> July/August 2012 Vol 12 No 6<br />
27