Triethylene Glycol - The Dow Chemical Company
Triethylene Glycol - The Dow Chemical Company
Triethylene Glycol - The Dow Chemical Company
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Properties<br />
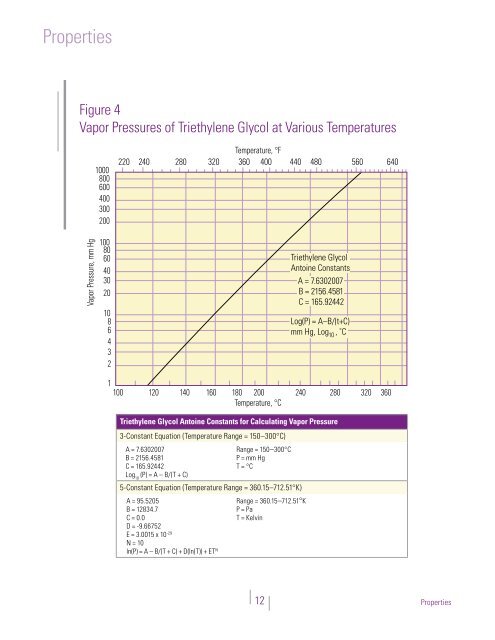
Figure 4: Vapor Pressures of <strong>Triethylene</strong> <strong>Glycol</strong><br />
at Various Temperatures<br />
Figure 4<br />
Vapor Pressures of <strong>Triethylene</strong> <strong>Glycol</strong> at Various Temperatures<br />
Vapor Pressure, mm Hg<br />
1000<br />
800<br />
600<br />
400<br />
300<br />
200<br />
100<br />
80<br />
60<br />
40<br />
30<br />
20<br />
10<br />
8<br />
6<br />
4<br />
3<br />
2<br />
Temperature, °F<br />
220 240 280 320 360 400 440 480 560 640<br />
1<br />
100 120 140 160 180 200 240 280 320 360<br />
Temperature, °C<br />
<strong>Triethylene</strong> <strong>Glycol</strong> Antoine Constants for Calculating Vapor Pressure<br />
<strong>Triethylene</strong> <strong>Glycol</strong> Antoine Constants for Calculating Vapor Pressure<br />
3-Constant Equation (Temperature Range = 150–300°C)<br />
3-Constant Equation A = 7 (Temperature 6302007 Range = 150 - 300°C) Range = 150–300°C<br />
A=<br />
7.<br />
63B<br />
0=<br />
22156<br />
00<br />
74581<br />
P = mm RHg<br />
ange<br />
= 150<br />
- 30<br />
0°<br />
C<br />
B=<br />
215<br />
6C<br />
. = 4165<br />
58192442<br />
T = °C P = mm<br />
H g<br />
Log (P) = A – B/(T + C)<br />
C = 165<br />
. 9210<br />
44<br />
2<br />
T = ° C<br />
Log 5-Constant 10 (P) = A – B/(T Equation + C) (Temperature Range = 360 15–712 51°K)<br />
A = 95 5205 Range = 360 15–712 51°K<br />
5-Constant Equation (Temperature Range = 360.15 - 712.51°K)<br />
A=<br />
95<br />
. 52<br />
05<br />
R ange<br />
= 36<br />
0.<br />
15<br />
- 712<br />
. 51K<br />
B=<br />
12<br />
83<br />
4.<br />
7<br />
P = Pa<br />
C = 0.<br />
0<br />
T = K elvin<br />
D = -9.6 6752<br />
E=3.0015 x 10 -29<br />
N=10<br />
ln(P) = A – B/(T + C) + D(ln(T)) + ET N<br />
B = 12834 7 P = Pa<br />
C = 0 0 T = Kelvin<br />
D = -9 66752<br />
E = 3 0015 x 10-29 N = 10<br />
ln(P) = A – B/(T + C) + D(ln(T)) + ETN 12<br />
<strong>Triethylene</strong> <strong>Glycol</strong><br />
Antoine Constants<br />
A = 7.6302007<br />
B = 2156.4581<br />
C = 165.92442<br />
Log(P) = A–B/(t+C)<br />
mm Hg, Log 10 , ˚C<br />
Properties