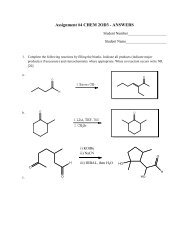
(TLC) and
(TLC) and
(TLC) and
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
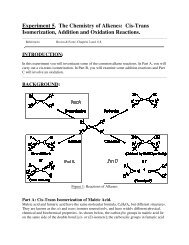
A layer of s<strong>and</strong> is placed over the top of the adsorbent, <strong>and</strong> the whole column is wetted with the<br />
solvent to be used. A solution of the substance to be purified in this solvent is then applied<br />
evenly to the top of the column, <strong>and</strong> this solution is allowed to pass down into the column so that<br />
the dissolved solid is adsorbed at the top of the column. The column is then eluted by passing<br />
down a number of solvents of increasing polarity. In this way, weakly adsorbed substances will<br />
pass rapidly through the column while the more strongly adsorbed substances will pass through<br />
at a slower rate. By eluting with a series of solvents of increasing polarity it is therefore possible<br />
to separate the components of a mixture <strong>and</strong> to elute them, one after the other, from the solid<br />
adsorbent.<br />
Choice of Adsorbent<br />
The order in which the compounds are eluted will depend on how strongly they are adsorbed on<br />
the surface of the stationary phase. A few adsorbents used in column chromatography, with<br />
different binding abilities are Cellulose, Calcium oxide, Silica gel (oxides of silicon), <strong>and</strong><br />
alumina (aluminum oxide). Alumina unless specially pretreated is slightly basic <strong>and</strong> hence<br />
strongly adsorbs acidic substances or materials capable of forming hydrogen bonds to the basic<br />
oxygen atoms of the alumina. Compounds without the ability to form hydrogen bonds but with<br />
substantial dipole moments will be somewhat less strongly adsorbed due to electronic<br />
interactions between their dipoles <strong>and</strong> those of the alumina. Compounds with neither acidic<br />
hydrogens nor dipole moments are only very weakly adsorbed due to dipole induced dipole<br />
interactions. The complete order for the strength of all these bonding interactions is generally the<br />
following: Salt Formation > Coordination Complexes > Hydrogen Bonding > Dipole-Dipole<br />
Interactions > Van der Waals. Some examples of these bonding interactions with alumina are<br />
shown:<br />
Choice of Solvent<br />
The choice of solvents used to elute the various components of the mixture from the column will<br />
depend upon the components in the mixture. For a very weakly adsorbed component a very nonpolar<br />
solvent such as petroleum ether or benzene would be used. For more strongly adsorbed<br />
components, a more polar solvent such as ether might be used. For very strongly adsorbed<br />
components, a very polar solvent such as ethanol or even acetic acid might be required to<br />
displace the material from the column. A list of common solvents in order of increasing eluting<br />
power follows:<br />
Petroleum Ether<br />
Carbon Tetrachloride<br />
Cyclohexane<br />
Carbon Disulfide<br />
(non-polar)<br />
increasing Benzene<br />
Toluene increasing<br />
eluting Methylene Chloride<br />
Chloroform polarity