Annex XV dossier PROPOSAL FOR ... - ECHA - Europa
Annex XV dossier PROPOSAL FOR ... - ECHA - Europa
Annex XV dossier PROPOSAL FOR ... - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANNEX <strong>XV</strong> – IDENTIFICATION OF SVHC – 2-ETHOXYETHANOL<br />
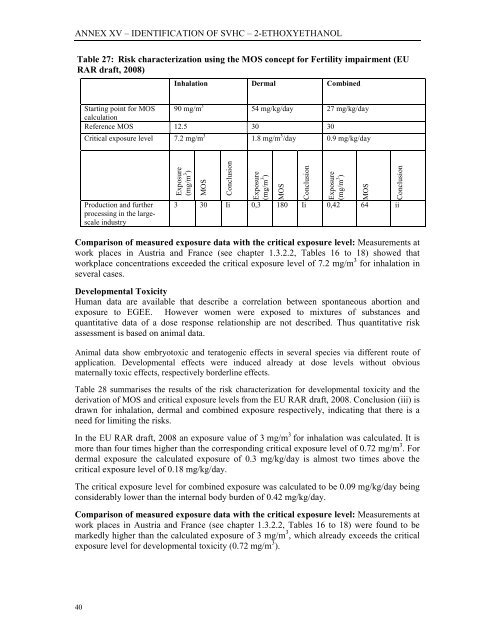
Table 27: Risk characterization using the MOS concept for Fertility impairment (EU<br />
RAR draft, 2008)<br />
40<br />
Inhalation Dermal Combined<br />
Starting point for MOS<br />
calculation<br />
90 mg/m 3 54 mg/kg/day 27 mg/kg/day<br />
Reference MOS 12.5 30 30<br />
Critical exposure level 7.2 mg/m 3 1.8 mg/m 3 /day 0.9 mg/kg/day<br />
Production and further<br />
processing in the largescale<br />
industry<br />
Exposure<br />
(mg/m 3 )<br />
MOS<br />
Conclusion<br />
Exposure<br />
(mg/m 3 )<br />
MOS<br />
Conclusion<br />
Exposure<br />
(mg/m 3 )<br />
3 30 Ii 0,3 180 Ii 0,42 64 ii<br />
Comparison of measured exposure data with the critical exposure level: Measurements at<br />
work places in Austria and France (see chapter 1.3.2.2, Tables 16 to 18) showed that<br />
workplace concentrations exceeded the critical exposure level of 7.2 mg/m 3 for inhalation in<br />
several cases.<br />
Developmental Toxicity<br />
Human data are available that describe a correlation between spontaneous abortion and<br />
exposure to EGEE. However women were exposed to mixtures of substances and<br />
quantitative data of a dose response relationship are not described. Thus quantitative risk<br />
assessment is based on animal data.<br />
Animal data show embryotoxic and teratogenic effects in several species via different route of<br />
application. Developmental effects were induced already at dose levels without obvious<br />
maternally toxic effects, respectively borderline effects.<br />
Table 28 summarises the results of the risk characterization for developmental toxicity and the<br />
derivation of MOS and critical exposure levels from the EU RAR draft, 2008. Conclusion (iii) is<br />
drawn for inhalation, dermal and combined exposure respectively, indicating that there is a<br />
need for limiting the risks.<br />
In the EU RAR draft, 2008 an exposure value of 3 mg/m 3 for inhalation was calculated. It is<br />
more than four times higher than the corresponding critical exposure level of 0.72 mg/m 3 . For<br />
dermal exposure the calculated exposure of 0.3 mg/kg/day is almost two times above the<br />
critical exposure level of 0.18 mg/kg/day.<br />
The critical exposure level for combined exposure was calculated to be 0.09 mg/kg/day being<br />
considerably lower than the internal body burden of 0.42 mg/kg/day.<br />
Comparison of measured exposure data with the critical exposure level: Measurements at<br />
work places in Austria and France (see chapter 1.3.2.2, Tables 16 to 18) were found to be<br />
markedly higher than the calculated exposure of 3 mg/m 3 , which already exceeds the critical<br />
exposure level for developmental toxicity (0.72 mg/m 3 ).<br />
MOS<br />
Conclusion