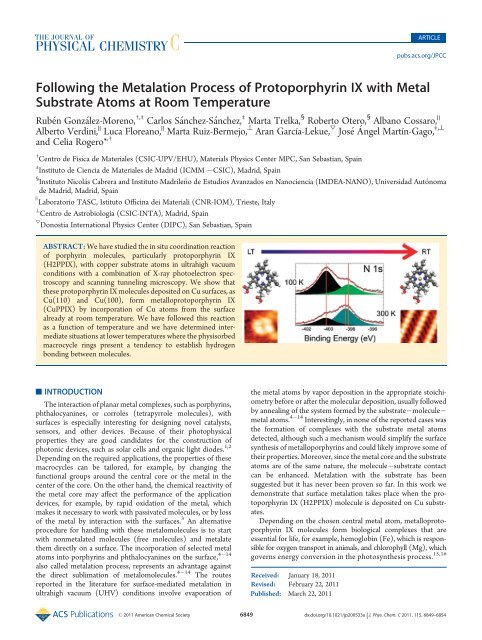
Following the Metalation Process of Protoporphyrin IX with Metal ...
Following the Metalation Process of Protoporphyrin IX with Metal ...
Following the Metalation Process of Protoporphyrin IX with Metal ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Received: January 18, 2011<br />
Revised: February 22, 2011<br />
Published: March 22, 2011<br />
ARTICLE<br />
pubs.acs.org/JPCC<br />
<strong>Following</strong> <strong>the</strong> <strong><strong>Metal</strong>ation</strong> <strong>Process</strong> <strong>of</strong> <strong>Protoporphyrin</strong> <strong>IX</strong> <strong>with</strong> <strong>Metal</strong><br />
Substrate Atoms at Room Temperature<br />
Ruben Gonzalez-Moreno, †,‡ Carlos Sanchez-Sanchez, ‡ Marta Trelka, § Roberto Otero, § Albano Cossaro, ||<br />
Alberto Verdini, || Luca Floreano, || Marta Ruiz-Bermejo, ^ Aran García-Lekue, 3 Jose Angel Martín-Gago, ‡,^<br />
and Celia Rogero* ,†<br />
†<br />
Centro de Física de Materiales (CSIC-UPV/EHU), Materials Physics Center MPC, San Sebastian, Spain<br />
‡<br />
Instituto de Ciencia de Materiales de Madrid (ICMM CSIC), Madrid, Spain<br />
§<br />
Instituto Nicolas Cabrera and Instituto Madrile~no de Estudios Avanzados en Nanociencia (IMDEA-NANO), Universidad Autonoma<br />
de Madrid, Madrid, Spain<br />
Laboratorio TASC, Istituto Officina dei Materiali (CNR-IOM), Trieste, Italy<br />
^<br />
Centro de Astrobiología (CSIC-INTA), Madrid, Spain<br />
3<br />
Donostia International Physics Center (DIPC), San Sebastian, Spain<br />
)<br />
ABSTRACT: We have studied <strong>the</strong> in situ coordination reaction<br />
<strong>of</strong> porphyrin molecules, particularly protoporphyrin <strong>IX</strong><br />
(H2PP<strong>IX</strong>), <strong>with</strong> copper substrate atoms in ultrahigh vacuum<br />
conditions <strong>with</strong> a combination <strong>of</strong> X-ray photoelectron spectroscopy<br />
and scanning tunneling microscopy. We show that<br />
<strong>the</strong>se protoporphyrin <strong>IX</strong> molecules deposited on Cu surfaces, as<br />
Cu(110) and Cu(100), form metalloprotoporphyrin <strong>IX</strong><br />
(CuPP<strong>IX</strong>) by incorporation <strong>of</strong> Cu atoms from <strong>the</strong> surface<br />
already at room temperature. We have followed this reaction<br />
as a function <strong>of</strong> temperature and we have determined intermediate<br />
situations at lower temperatures where <strong>the</strong> physisorbed<br />
macrocycle rings present a tendency to establish hydrogen<br />
bonding between molecules.<br />
’ INTRODUCTION<br />
The interaction <strong>of</strong> planar metal complexes, such as porphyrins,<br />
phthalocyanines, or corroles (tetrapyrrole molecules), <strong>with</strong><br />
surfaces is especially interesting for designing novel catalysts,<br />
sensors, and o<strong>the</strong>r devices. Because <strong>of</strong> <strong>the</strong>ir photophysical<br />
properties <strong>the</strong>y are good candidates for <strong>the</strong> construction <strong>of</strong><br />
photonic devices, such as solar cells and organic light diodes. 1,2<br />
Depending on <strong>the</strong> required applications, <strong>the</strong> properties <strong>of</strong> <strong>the</strong>se<br />
macrocycles can be tailored, for example, by changing <strong>the</strong><br />
functional groups around <strong>the</strong> central core or <strong>the</strong> metal in <strong>the</strong><br />
center <strong>of</strong> <strong>the</strong> core. On <strong>the</strong> o<strong>the</strong>r hand, <strong>the</strong> chemical reactivity <strong>of</strong><br />
<strong>the</strong> metal core may affect <strong>the</strong> performance <strong>of</strong> <strong>the</strong> application<br />
devices, for example, by rapid oxidation <strong>of</strong> <strong>the</strong> metal, which<br />
makes it necessary to work <strong>with</strong> passivated molecules, or by loss<br />
<strong>of</strong> <strong>the</strong> metal by interaction <strong>with</strong> <strong>the</strong> surfaces. 3 An alternative<br />
procedure for handling <strong>with</strong> <strong>the</strong>se metalomolecules is to start<br />
<strong>with</strong> nonmetalated molecules (free molecules) and metalate<br />
<strong>the</strong>m directly on a surface. The incorporation <strong>of</strong> selected metal<br />
atoms into porphyrins and phthalocyanines on <strong>the</strong> surface,<br />
4 14<br />
also called metalation process, represents an advantage against<br />
<strong>the</strong> direct sublimation <strong>of</strong> metalomolecules. 4 14 The routes<br />
reported in <strong>the</strong> literature for surface-mediated metalation in<br />
ultrahigh vacuum (UHV) conditions involve evaporation <strong>of</strong><br />
<strong>the</strong> metal atoms by vapor deposition in <strong>the</strong> appropriate stoichiometry<br />
before or after <strong>the</strong> molecular deposition, usually followed<br />
by annealing <strong>of</strong> <strong>the</strong> system formed by <strong>the</strong> substrate molecule<br />
metal atoms. 4 14 Interestingly, in none <strong>of</strong> <strong>the</strong> reported cases was<br />
<strong>the</strong> formation <strong>of</strong> complexes <strong>with</strong> <strong>the</strong> substrate metal atoms<br />
detected, although such a mechanism would simplify <strong>the</strong> surface<br />
syn<strong>the</strong>sis <strong>of</strong> metalloporphyrins and could likely improve some <strong>of</strong><br />
<strong>the</strong>ir properties. Moreover, since <strong>the</strong> metal core and <strong>the</strong> substrate<br />
atoms are <strong>of</strong> <strong>the</strong> same nature, <strong>the</strong> molecule substrate contact<br />
can be enhanced. <strong><strong>Metal</strong>ation</strong> <strong>with</strong> <strong>the</strong> substrate has been<br />
suggested but it has never been proven so far. In this work we<br />
demonstrate that surface metalation takes place when <strong>the</strong> protoporphyrin<br />
<strong>IX</strong> (H2PP<strong>IX</strong>) molecule is deposited on Cu substrates.<br />
Depending on <strong>the</strong> chosen central metal atom, metalloprotoporphyrin<br />
<strong>IX</strong> molecules form biological complexes that are<br />
essential for life, for example, hemoglobin (Fe), which is responsible<br />
for oxygen transport in animals, and chlorophyll (Mg), which<br />
governs energy conversion in <strong>the</strong> photosyn<strong>the</strong>sis process. 15,16<br />
r 2011 American Chemical Society 6849 dx.doi.org/10.1021/jp200533a | J. Phys. Chem. C 2011, 115, 6849–6854
The Journal <strong>of</strong> Physical Chemistry C ARTICLE<br />
Figure 1. (a) Top and (B) side views <strong>of</strong> <strong>the</strong> DFT-optimized ball-andstick<br />
model <strong>of</strong> H2PP<strong>IX</strong>.<br />
The latter feature is one <strong>of</strong> <strong>the</strong> main reasons why this family <strong>of</strong><br />
molecules is investigated <strong>with</strong>in <strong>the</strong> context <strong>of</strong> photoluminescence<br />
applications. The H2PP<strong>IX</strong> molecule is <strong>the</strong> nonmetalated<br />
version <strong>of</strong> this family <strong>of</strong> molecules, where two hydrogen atoms<br />
replace <strong>the</strong> central metal one. Its structure is almost planar and<br />
<strong>the</strong> central macrocycle is surrounded only by short methyl and<br />
e<strong>the</strong>nyl groups and two propionic acids (Figure 1).<br />
In this work we use X-ray photoelectron spectroscopy (XPS)<br />
to investigate <strong>the</strong> chemical changes observed in H2PP<strong>IX</strong> when<br />
<strong>the</strong> molecule is interacting directly <strong>with</strong> metal surfaces, as<br />
Cu(110) and Cu(100). These surfaces have been chosen as<br />
models for fur<strong>the</strong>r studies on more realistic ones for applications,<br />
as <strong>the</strong>y could be metallic oxides. We study <strong>the</strong> transition from <strong>the</strong><br />
physisorbed molecular configuration to <strong>the</strong> chemisorbed one as a<br />
function <strong>of</strong> <strong>the</strong> temperature. Thus, we follow <strong>the</strong> coordination<br />
reaction <strong>with</strong> <strong>the</strong> substrate atoms, demonstrating that, already at<br />
room temperature, H2PP<strong>IX</strong> molecules adsorbed on Cu surfaces<br />
can form Cu-protoporphyrin <strong>IX</strong> (CuPP<strong>IX</strong>) in strongly bound<br />
self-assembled monolayers (SAMs), as confirmed by scanning<br />
tunneling microscopy (STM) images.<br />
’ EXPERIMENTAL DETAILS<br />
Growth <strong>of</strong> Molecules. The growth <strong>of</strong> <strong>the</strong> molecular films is<br />
performed in UHV, <strong>with</strong> a base pressure <strong>of</strong>
The Journal <strong>of</strong> Physical Chemistry C ARTICLE<br />
Figure 2. N1s core-level XPS peaks <strong>of</strong> (A) bulk reference H2PP<strong>IX</strong> molecules; (B) H2PP<strong>IX</strong> evaporated on Cu(100) at 160 K and annealed to RT; (C)<br />
H2PP<strong>IX</strong> evaporated on Cu(110) at 200 K and annealed to RT; (D) H2PP<strong>IX</strong> evaporated on Cu(110) at 200 K and annealed at 573 K for 3 min; (E)<br />
H2PP<strong>IX</strong> evaporated on Cu(110) at 263 K; (F, G) H2PP<strong>IX</strong> evaporated on Cu(110) at 200 K (F, monolayer; G, low coverage); and (H) reference<br />
molecule in solution in DMCO.<br />
Figure 2 A shows <strong>the</strong> N1s core level peak <strong>of</strong> <strong>the</strong> powder<br />
H2PP<strong>IX</strong> molecule used as reference and <strong>the</strong> best fit obtained.<br />
The spectrum shows two equivalent components as expected for<br />
<strong>the</strong> pristine molecule <strong>with</strong> <strong>the</strong> two nonequivalent N atoms,<br />
iminic at 397.8 eV and pyrrolic at 399.9 eV (47% and 53% <strong>of</strong><br />
<strong>the</strong> total intensity, respectively). The line shape <strong>of</strong> N 1s changes<br />
completely when <strong>the</strong> molecules are deposited on <strong>the</strong> copper<br />
substrates. Figure 2 panels B and C show <strong>the</strong> N 1s core level<br />
measured on Cu(100) and Cu(110) substrates, respectively,<br />
after evaporation <strong>of</strong> H2PP<strong>IX</strong> at low temperature and <strong>the</strong><br />
subsequent anneal at RT (<strong>the</strong> same result is obtained directly<br />
evaporating at RT, although <strong>with</strong> much lower sticking <strong>of</strong> <strong>the</strong><br />
molecule). In both cases <strong>the</strong> intensity <strong>of</strong> <strong>the</strong> component at higher<br />
binding energy clearly decreases and <strong>the</strong> spectrum presents an<br />
intense component at 398.2 eV, slightly shifted <strong>with</strong> respect to<br />
<strong>the</strong> 397.8 eV <strong>of</strong> <strong>the</strong> iminic groups.<br />
The origin <strong>of</strong> this new line shape can be explained as <strong>the</strong> result<br />
<strong>of</strong> <strong>the</strong> reaction <strong>of</strong> <strong>the</strong> four N atoms <strong>with</strong> <strong>the</strong> Cu substrate atoms<br />
forming a coordination bond; that is, metalation. In fact, this<br />
component is not at <strong>the</strong> same position <strong>of</strong> <strong>the</strong> iminic N in <strong>the</strong><br />
reference (we remark that it is not possible to have molecules<br />
<strong>with</strong> <strong>the</strong> four N atoms in an iminic conformation). Its energy<br />
position is consistent <strong>with</strong> <strong>the</strong> nitrogen binding energy reported<br />
for o<strong>the</strong>r copper porphyrins. 21 Compared to o<strong>the</strong>r bulk<br />
metalloporphyrins, 10,22 <strong>the</strong> energy position for <strong>the</strong> CuPP<strong>IX</strong> film<br />
is slightly lower although compatible <strong>with</strong> <strong>the</strong>m, if we take into<br />
account two effects related to <strong>the</strong> planar geometry <strong>of</strong> <strong>the</strong><br />
molecule and <strong>the</strong> submonolayer thickness: (i) substrate charge<br />
transfer and (ii) a more efficient screening <strong>of</strong> <strong>the</strong> final core hole.<br />
In some cases, <strong>the</strong> first stage <strong>of</strong> <strong>the</strong> metalation is witnessed by <strong>the</strong><br />
appearance <strong>of</strong> a small component at 399.1 eV. This component is<br />
attributed to deprotonated polypyrroles, that is, macrocycles that<br />
have lost one H atom, 23 and it is an intermediate step before <strong>the</strong><br />
coordination reaction. No variation <strong>of</strong> <strong>the</strong> Cu photoemission<br />
spectra was detected, because <strong>of</strong> <strong>the</strong> very low surface density <strong>of</strong><br />
incorporated Cu atoms even when our measurements are<br />
performed at grazing incidence.<br />
The feasibility <strong>of</strong> in situ metalation <strong>of</strong> preadsorbed metal-free<br />
tetrapyrroles <strong>with</strong> vapor-deposited metal atoms was initially<br />
demonstrated for Fe 13,14 and extended later for o<strong>the</strong>r metals,<br />
such as Ce, Zn, Co, or Ni. 5 8,24 For some <strong>of</strong> <strong>the</strong>m, like Fe and Co,<br />
<strong>the</strong> reaction proceeds also at RT. For o<strong>the</strong>rs, like Zn, 8 <strong>the</strong><br />
reaction requires elevated temperatures (above 500 K). On <strong>the</strong><br />
basis <strong>of</strong> density functional <strong>the</strong>oery (DFT) calculations <strong>of</strong> <strong>the</strong><br />
activation barriers, Cu and Zn were proposed to react <strong>with</strong><br />
porphyrins at elevated temperatures. 8 In <strong>the</strong> present case, we<br />
observed no significant changes ei<strong>the</strong>r in <strong>the</strong> position or in <strong>the</strong><br />
shape <strong>of</strong> <strong>the</strong> core level after an annealing beyond room temperature.<br />
Figure 2 D shows <strong>the</strong> spectrum <strong>of</strong> <strong>the</strong> molecules absorbed<br />
on Cu(110) and measured after an annealing <strong>of</strong> <strong>the</strong> system at 573<br />
6851 dx.doi.org/10.1021/jp200533a |J. Phys. Chem. C 2011, 115, 6849–6854
The Journal <strong>of</strong> Physical Chemistry C ARTICLE<br />
Figure 3. O 1s core level XPS peaks <strong>of</strong> H2PP<strong>IX</strong>, evaporated on (A)<br />
Cu(110) at 200 K and (B) Cu(100) at 160 K.<br />
K (same behavior is observed for <strong>the</strong> o<strong>the</strong>r crystal face). At higher<br />
temperature, <strong>the</strong> molecule starts to decompose. In fact, <strong>the</strong> signal<br />
<strong>of</strong> <strong>the</strong> O 1s core level disappears and new components in <strong>the</strong> C 1s<br />
core level appear at lower binding energies that are related to<br />
Cu C interactions. Therefore, contrary to <strong>the</strong> <strong>the</strong>oretical predictions<br />
calculated for added adatoms, <strong>the</strong> H2PP<strong>IX</strong> molecules<br />
metalate <strong>with</strong> <strong>the</strong> Cu substrate atoms already at RT. Moreover,<br />
we have observed that this process starts at even lower temperatures<br />
. When <strong>the</strong> evaporation is performed <strong>with</strong> <strong>the</strong> substrate held<br />
at 263K (see Figure 2 E), <strong>the</strong> N 1s core level presents one<br />
relevant component centered at 398.2 eV, which is related to <strong>the</strong><br />
metalated component ra<strong>the</strong>r than to <strong>the</strong> iminic groups. Ano<strong>the</strong>r<br />
component is visible at 399.9 eV that might be entirely attributed<br />
to <strong>the</strong> pyrrolic N. This double component assignment would<br />
suggest an intermediate situation, <strong>with</strong> coexisting free and<br />
metalated molecules. In fact, similar evidence was reported for<br />
<strong>the</strong> metalation <strong>of</strong> tetraphenylporphyrin (H2TPP) on Ag(111) by<br />
evaporation <strong>of</strong> Zn 4,8 where, at RT, <strong>the</strong> coexistence <strong>of</strong> three<br />
species was reported: (i) free molecules, (ii) metalloporphyrins,<br />
and (iii) an intermediate species where <strong>the</strong> Zn atoms were<br />
coordinated although <strong>the</strong> pyrrolic N H bonds were preserved.<br />
However, such a simplified decomposition <strong>of</strong> <strong>the</strong> spectrum in<br />
Figure 2E is in contrast <strong>with</strong> <strong>the</strong> molecular stoichiometry, since it<br />
would indicate an excess <strong>of</strong> <strong>the</strong> pyrrolic component <strong>with</strong> respect<br />
to <strong>the</strong> iminic one for <strong>the</strong> population <strong>of</strong> <strong>the</strong> intact metal-free<br />
molecules.<br />
In order to understand <strong>the</strong> origin <strong>of</strong> this apparently unbalanced<br />
component, we study <strong>the</strong> behavior <strong>of</strong> <strong>the</strong>se molecules<br />
when <strong>the</strong>y just “land” at LT, just after <strong>the</strong> deposition. Figure 2 F<br />
shows a high-resolution spectrum <strong>of</strong> N 1s core level after <strong>the</strong><br />
evaporation <strong>of</strong> a submonolayer <strong>of</strong> H2PP<strong>IX</strong> on Cu(110) <strong>with</strong> <strong>the</strong><br />
substrate at 200 K. The intensity <strong>of</strong> <strong>the</strong> iminic component almost<br />
disappears and <strong>the</strong> spectrum presents an intense component at<br />
400.1 eV, toge<strong>the</strong>r <strong>with</strong> a small deprotonated component at<br />
399.1 eV and an even fainter component at 397.9 eV (this<br />
component is relatively larger for very low coverage). Apart from<br />
<strong>the</strong> slightly larger binding energy (þ0.2 eV), <strong>the</strong> energy <strong>of</strong> <strong>the</strong><br />
former component would be compatible <strong>with</strong> most <strong>of</strong> <strong>the</strong> N<br />
atoms in a pyrrolic-like configuration. This might imply that <strong>the</strong><br />
molecule surface interaction mediates <strong>the</strong> formation <strong>of</strong> a<br />
Figure 4. (A, B) N 1s and (C, D) O 1s core-level XPS peaks <strong>of</strong> ZnPP<strong>IX</strong>,<br />
(A, C) evaporated on Cu(110) at 220 K and (B, D) annealed to RT.<br />
zwitterionic phase <strong>of</strong> almost all molecules where <strong>the</strong> H atoms<br />
from <strong>the</strong> carboxylic acid groups migrate to <strong>the</strong> center <strong>of</strong> <strong>the</strong><br />
pyrrole rings. 3<br />
To check <strong>the</strong> possible occurrence <strong>of</strong> this change <strong>of</strong> molecular<br />
charge state, we measured <strong>the</strong> XPS spectra from carboxylic<br />
groups <strong>of</strong> <strong>the</strong> molecule after evaporation. Figure 3 shows <strong>the</strong><br />
O 1s core level spectra measured on both samples, Cu(110) and<br />
Cu(100), at low temperature. Both spectra can be fitted <strong>with</strong><br />
three components, centered at 532.9, 531.8, and 530.9 eV. The<br />
components at 532.9 and 531.8 eV correspond to <strong>the</strong> two<br />
nonequivalent O atoms in <strong>the</strong> carboxylic acid group, CdO and<br />
C—OH, respectively, and <strong>the</strong> one at 530.9 eV corresponds to <strong>the</strong><br />
oxygen atoms in <strong>the</strong> deprotonated COO group. 25 27 Thus,<br />
although we have detected a partial deprotonation <strong>of</strong> <strong>the</strong><br />
carboxylic group at low temperature, its amount is not large<br />
enough to account for <strong>the</strong> dominant pyrrolic-like component in<br />
<strong>the</strong> N 1s core level around 400 eV.<br />
The hypo<strong>the</strong>sis <strong>of</strong> migration <strong>of</strong> H atoms from <strong>the</strong> carboxylic<br />
groups to <strong>the</strong> central core was proposed by Rienzo et al. 3 for <strong>the</strong><br />
adsorption <strong>of</strong> zinc protoporphyrin (ZnPtP) on TiO2 (110). In<br />
this work, <strong>the</strong> authors suggested that <strong>the</strong> strong interaction <strong>of</strong><br />
ZnPtP <strong>with</strong> <strong>the</strong> oxide surface induces <strong>the</strong> ejection <strong>of</strong> Zn atoms<br />
from <strong>the</strong> central core and <strong>the</strong> subsequent occupation <strong>with</strong> two H<br />
atoms probably comes from <strong>the</strong> carboxylic anchor groups. In<br />
order to check whe<strong>the</strong>r this mechanism can be operative on a<br />
6852 dx.doi.org/10.1021/jp200533a |J. Phys. Chem. C 2011, 115, 6849–6854
The Journal <strong>of</strong> Physical Chemistry C ARTICLE<br />
Figure 5. STM images <strong>of</strong> H2PP<strong>IX</strong>, (A) after evaporation on Cu(110) at<br />
200 K (100 100 nm 2 <strong>with</strong> a zoom <strong>of</strong> an individual molecule) and (B)<br />
after annealing to 300 K (5 5nm 2 ).<br />
copper substrate, we have performed comparative experiments<br />
<strong>with</strong> ZnPtP molecules deposited on <strong>the</strong> Cu(110) surface.<br />
Figure 4 panels A and C show <strong>the</strong> N 1s and O 1s core levels<br />
measured after evaporation <strong>of</strong> a submonolayer <strong>of</strong> ZnPtP on<br />
Cu(110) at LT (220 K). In <strong>the</strong> N 1s core level at LT, we observe<br />
for Cu(110) <strong>the</strong> same double peak result that Rienzo et al. 3<br />
obtained at RT on TiO 2 (110). However, at LT <strong>the</strong> O 1s core<br />
level presents a very low degree <strong>of</strong> deprotonation <strong>of</strong> <strong>the</strong><br />
carboxylic group. When <strong>the</strong> analysis is done after <strong>the</strong> annealing<br />
to RT, deprotonation <strong>of</strong> <strong>the</strong> carboxylic group is completed<br />
(Figure 4 D), although at this temperature we observed only<br />
<strong>the</strong> metalated molecule, <strong>with</strong> no signal in <strong>the</strong> pyrrolic N region<br />
(Figure 4 B). Therefore, even when <strong>the</strong> deprotonation <strong>of</strong> <strong>the</strong><br />
carboxylic group takes place and it is completed at RT, <strong>the</strong> high<br />
intensity <strong>of</strong> <strong>the</strong> component at around 400 eV in <strong>the</strong> N 1s core<br />
level cannot be related only to formation <strong>of</strong> <strong>the</strong> zwitterionic<br />
phase.<br />
An alternative explanation, which fits all our experimental<br />
evidences, is <strong>the</strong> formation <strong>of</strong> hydrogen-bond interactions between<br />
H in <strong>the</strong> carboxylic, methyl and/or e<strong>the</strong>nyl groups and N<br />
in <strong>the</strong> tetrapyrrole ring among surrounding molecules. For<br />
isonicotinic acid (where each molecule contains a single N<br />
atom), it has been reported by XPS that two N components<br />
occur in <strong>the</strong> N 1s core level that are separated by 1.7 eV. 28 This<br />
core-level shift is associated <strong>with</strong> hydrogen bonding between <strong>the</strong><br />
carboxylic group <strong>of</strong> one molecule and <strong>the</strong> nitrogen <strong>of</strong> <strong>the</strong><br />
pyridine ring <strong>of</strong> <strong>the</strong> next (this shift between <strong>the</strong> H-bonded<br />
nitrogen and <strong>the</strong> non-H-bonded N atoms is even higher, 1.9<br />
eV, for picolinic acid, which presents <strong>the</strong> same chemical formula<br />
but a different arrangement <strong>of</strong> atoms in <strong>the</strong> molecule). 29 This<br />
head-to-tail hydrogen bonding does not yield deprotonation <strong>of</strong><br />
<strong>the</strong> carboxylic group and is known from X-ray crystallography<br />
studies to connect <strong>the</strong> isonicotinic acid molecules in infinite<br />
chains in <strong>the</strong> solid state. 28 30 This mechanism might also apply<br />
to <strong>the</strong> present case, as favored by <strong>the</strong> planar orientation <strong>of</strong> <strong>the</strong><br />
molecules, large size <strong>of</strong> <strong>the</strong> hole in <strong>the</strong> center <strong>of</strong> <strong>the</strong> macrocycle,<br />
and flexibility <strong>of</strong> <strong>the</strong> bonds <strong>of</strong> <strong>the</strong> propionic acids. For comparison,<br />
we performed a reference experiment consisting <strong>of</strong> dissolving<br />
H2PP<strong>IX</strong> in a solvent, particularly DMSO. Figure 2 H shows<br />
<strong>the</strong> N 1s core level <strong>of</strong> <strong>the</strong> reference sample (check <strong>the</strong> Experimental<br />
Details section for specifics). It presents two components at<br />
binding energies <strong>of</strong> 399.9 and 397.8 eV. Also in this case part <strong>of</strong> <strong>the</strong><br />
iminic N atoms form <strong>the</strong> H bonding and <strong>the</strong> carboxylic group is not<br />
deprotonated, according to <strong>the</strong> double peak structure observed for<br />
<strong>the</strong> O 1s core level. We may conclude that H bonds between N and<br />
H atoms <strong>of</strong> adjacent molecules can be formed also by deposition<br />
from solution, in agreement <strong>with</strong> <strong>the</strong> mechanism observed by<br />
evaporation in UHV. We can speculate that <strong>the</strong> very low deposition<br />
rate <strong>of</strong> H2PP<strong>IX</strong> favors hydrogen bonding, as was also reported for<br />
<strong>the</strong> film growth <strong>of</strong> isonicotinic acid and its isomers. 31<br />
Finally, we remark that metalation is possible thanks to <strong>the</strong> close<br />
planarity <strong>of</strong> <strong>the</strong> molecular structure that allows <strong>the</strong> tetrapyrrole core<br />
to stay very close to <strong>the</strong> surface. Near-edge X-ray absorption fine<br />
structure (NEXAFS) and STM experiments confirm this assumption<br />
(a detailed discussion about <strong>the</strong> adsorption geometry will be <strong>the</strong><br />
object <strong>of</strong> ano<strong>the</strong>r work). Figure 5 A shows two STM images<br />
corresponding to H2PP<strong>IX</strong> deposited at low temperature on Cu-<br />
(110). After evaporation at 200 K, <strong>the</strong> molecules aggregates into a<br />
large density <strong>of</strong> small clusters, where molecules mainly lie parallel to<br />
<strong>the</strong> surface, as shown in <strong>the</strong> inset image <strong>of</strong> an isolated molecule. After<br />
annealing to RT, <strong>the</strong> metalation reaction is accompanied by selforganization<br />
<strong>of</strong> CuPP<strong>IX</strong>. Figure 5 B illustrates <strong>the</strong> organized<br />
structure observed after annealing to RT. In this molecular arrangement,<br />
variable-polarization NEXAFS measurements indicate PP<strong>IX</strong><br />
molecules to be oriented <strong>with</strong> <strong>the</strong> macrocycle almost parallel to <strong>the</strong><br />
surface. Porphyrin orientation induced by some kind <strong>of</strong> coordination<br />
reaction <strong>with</strong> <strong>the</strong> Cu substrate atoms has been previously<br />
reported. 22 Klappenberger et al. 23 discuss <strong>the</strong> formation <strong>of</strong> an<br />
ordered network <strong>of</strong> molecules mediated by <strong>the</strong> coordination<br />
reaction that takes place after evaporation and subsequent annealing<br />
<strong>of</strong> <strong>the</strong> tetrapyridylporphyrins (TPyP) on Cu(111). 23,32 In that case,<br />
<strong>the</strong> reaction affected <strong>the</strong> four peripheral N-pyridyl substituents<br />
instead <strong>of</strong> <strong>the</strong> four N atoms in <strong>the</strong> central macrocycle, and <strong>the</strong>refore<br />
metalation was not involved. 23 ThereasonisthatinTPyP<strong>the</strong><br />
pyridyl nitrogen atoms are not coplanar <strong>with</strong> <strong>the</strong> tetrapyrrole, and<br />
when <strong>the</strong> molecules interact <strong>with</strong> <strong>the</strong> surface, <strong>the</strong> central macrocycle<br />
is too far from <strong>the</strong> surface to react <strong>with</strong> it. 23,32 On <strong>the</strong> contrary, <strong>the</strong><br />
geometry <strong>of</strong> H2PP<strong>IX</strong> exhibiting an almost planar configuration<br />
allows <strong>the</strong> surface macrocycle interaction. We remark that direct<br />
metalation <strong>with</strong> surface atoms has not been reported yet for o<strong>the</strong>r<br />
planar tetrapyrroles (like octaethylporphyrin), possibly because <strong>the</strong>y<br />
have been mostly studied on Ag(111), 11 where<strong>the</strong>largesizemay<br />
result in an additional steric barrier. In fact, in UHV, no previous<br />
work reports such a clear surface metalation <strong>of</strong> any porphyrin. Only<br />
for gold substrate in a liquid environment a partial surface coordination<br />
<strong>of</strong> tetrapyrrole ring <strong>with</strong> <strong>the</strong> substrate was suggested, by<br />
Katsonis et al., 33 who pointed out that some iminic nitrogen atoms<br />
react <strong>with</strong> Au atoms thanks to <strong>the</strong> strong distortion that tetradodecylporphyrin<br />
suffers on Au(111).<br />
’ CONCLUSIONS<br />
In summary, we show a ready molecule substrate coordination<br />
reaction at RT leading to metalation <strong>of</strong> porphyrin molecules<br />
6853 dx.doi.org/10.1021/jp200533a |J. Phys. Chem. C 2011, 115, 6849–6854
The Journal <strong>of</strong> Physical Chemistry C ARTICLE<br />
(H2PP<strong>IX</strong>) <strong>with</strong> Cu surface atoms (CuPP<strong>IX</strong>). From our point <strong>of</strong><br />
view, <strong>the</strong>re are several factors that combine to make this reaction<br />
possible: (1) <strong>the</strong> closely planar structure <strong>of</strong> H2PP<strong>IX</strong>, which<br />
allows direct surface tetrapyrrole ring contact; 34 (2) <strong>the</strong> high<br />
density <strong>of</strong> Cu adatoms moving on <strong>the</strong> crystal surfaces that could<br />
be involved in <strong>the</strong> coordination reaction; 10 and (3) <strong>the</strong> rapid rate<br />
<strong>of</strong> incorporation <strong>of</strong> Cu ions by porphyrin macrocycles 35 and <strong>the</strong><br />
high deprotonation rate at RT. The coordinated porphyrins form<br />
ordered self-assembled monolayers upon annealing. This highly<br />
stable porphyrin network is more suitable for applications than<br />
previously attempts to build SAMs <strong>with</strong> porphyrins.<br />
’ AUTHOR INFORMATION<br />
Corresponding Author<br />
*Centro de Física de Materiales, Po. Manuel de Lardizabal 5,<br />
Donostia - San Sebastian, Gipuzkoa E-20018, Spain. Tel (þ34)<br />
943015804; fax (þ34) 943015800; e-mail celia_rogero@ehu.es.<br />
’ ACKNOWLEDGMENT<br />
We acknowledge funding through Spanish research projects<br />
CSD2007-41, MAT2008-1497, PET2008-109, FIS2010-19609-<br />
C02-00, and BIO 2007-67523 and intramural project 200960I159.<br />
C.S.-S. is grateful to Ministerio de Educacion for <strong>the</strong> AP2005-0433<br />
FPU grant. C.R. and R.G.-M. acknowledge help from and useful<br />
discussions <strong>with</strong> A. Arnau and Enrique Ortega.<br />
’ REFERENCES<br />
(1) Maree, C. H. M.; Roosendaal, S. J.; Savenije, T. J.; Schropp,<br />
R. E. I.; Schaafsma, T. J.; Habraken, F. H. P. M. J. Appl. Phys. 1996,<br />
80, 3381.<br />
(2) Harima, Y.; Okazaki, H.; Kunugi, Y.; Yamashita, K.; Ishii, H.;<br />
Seki, K. Appl. Phys. Lett. 1996, 69, 1059.<br />
(3) Rienzo, A.; Mayor, L. C.; Magnano, G.; Satterley, C. J.; O’Shea,<br />
J. N.; Ataman, E.; Schnadt, J.; Schulte, K. J. Chem. Phys. 2010, 132, No.<br />
084703.<br />
(4) Gottfried, J. M.; Marbach, H. Z. Phys. Chem. 2009, 223, 53.<br />
(5) Weber-Bargioni, A.; Reichert, J.; Seitsonen, A. P.; Auw€arter, W.;<br />
Schiffrin, A.; Barth, J. V. Phys. Chem. C 2008, 112, 3453.<br />
(6) Kretschmann, A.; Walz, M.-M.; Flechtner, K.; Steinr€uck, H.-P.;<br />
Gottfried, J. M. Chem. Commun. 2007, 568.<br />
(7) Gottfried, J. M.; Flechtner, K.; Kretschmann, A.; Lukasczyk, Th.;<br />
Steinr€uck, H.-P. J. Am. Chem. Soc. 2006, 128, 5644.<br />
(8) Shubina, T. E.; Marbach, H.; Flechtner, K.; Kretschmann, A.; Jux,<br />
N.; Buchner, F.; Steinr€uck, H.-P.; Clark, T.; Gottfried, J. M. J. Am. Chem.<br />
Soc. 2007, 129, 9476.<br />
(9) Buchner, F.; Warnick, K. G.; Wolfle, T.; Gorling, A.; Steinruck,<br />
H. P.; Hieringer, W.; Marbach, H. J. Phys. Chem. C 2009, 113, 16450.<br />
(10) Buchner, F.; Flechtner, K.; Bai, Y.; Zillner, E.; Kellner, I.;<br />
Steinr€uck, H.-P.; Marbach, H.; Gottfried, J. M. J. Phys. Chem. C 2008,<br />
112, 15458.<br />
(11) Bai, Y.; Buchner, F.; Wendahl, M. T.; Kellner, I.; Bayer, A.;<br />
Steinr€uck, H.-P.; Marbach, H.; Gottfried, J. M. J. Phys. Chem. C 2008,<br />
112, 6087.<br />
(12) Ecija, D.; Trelka, M.; Urban, C.; de Mendoza, P.; Mateo-Marti,<br />
E.; Rogero, C.; Martin-Gago, J. A.; Echavarren, A. M.; Otero, R.; Gallego,<br />
J. M.; Miranda, R. BiochemistryJ. Phys. Chem. C 2008, 112, 8988.<br />
(13) Auw€arter, W.; Weber-Bargioni, A.; Brink, S.; Riemann, A.;<br />
Schiffrin, A.; Ruben, M.; Barth, J. V. Chem. Phys. Chem. 2007, 8, 250.<br />
(14) Buchner, F.; Schwald, V.; Comanici, K.; Steinruck, H. P.;<br />
Marbach, H. ChemPhysChem 2007, 8, 241.<br />
(15) Voet, D.; Voet, J. G. Biochemistry, 3rd ed.; John Wiley & Sons<br />
Inc.: New York, 2004.<br />
(16) van Grondelle, R.; Dekker, J. P.; Gillbro, T.; Sundstrom, V.<br />
Biochim. Biophys. Acta 1994, 1, 1187.<br />
(17) Moulder, J. F.; Stickle, W. F.; Sobol, P. E.; Bomben, K. D.<br />
Handbook <strong>of</strong> X-ray Pholoelectron Spectroscopy; PerkinElmer Corp.:<br />
Waltham, MA, 1992.<br />
(18) Cossaro, A.; Floreano, L.; Verdini, A.; Casalis, L.; Morgante, A.<br />
Phys. Rev. Lett. 2009, 103, No. 119601.<br />
(19) Floreano, L.; Cossaro, A.; Gotter, R.; Verdini, A.; Bavdek, G.;<br />
Evangelista, F.; Ruocco, A.; Morgante, A.; Cvetko, D. J. Phys. Chem. C<br />
2008, 112, 10794.<br />
(20) Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J. M.; Colchero,<br />
J.; Gomez-Herrero, J.; Baro, A. M. Rev. Sci. Instrum. 2007, 78, No.<br />
013705.<br />
(21) Gulino, A.; Fragala, I.; Scamporrino, E.; Vitalini, D. J. Phys.<br />
Chem. C 2007, 111, 14125.<br />
(22) Mele, G.; Del Sole, R.; Vasapollo, G.; Marci, G.; Garcia-Lopez,<br />
E.; Palmisano, L.; Coronado, J. M.; Hernandez-Alonso, M. D.; Malitesta,<br />
C.; Guascito, M. R. J. Phys. Chem. B 2005, 109, 12347.<br />
(23) Klappenberger, F.; Weber-Bargioni, A.; Marschall, M.;<br />
Schiffrin, A.; Auw€arter, W.; Barth, J. V. J. Chem. Phys. 2008, 129, No.<br />
214702.<br />
(24) Chen, M.; Feng, X.; Zhang, L.; Ju, H.; Xu, Q.; Zhu, J.; Gotffried,<br />
J. M.; Ibrahim, K.; Qian, H.; Wang, J. J. Phys Chem. C 2010, 114, 9908.<br />
(25) Stepanow, S.; Strunskus, T.; Lingenfelder, M.; Dmitriev, A.;<br />
Spillmann, H.; Lin, N.; Barth, J. V.; W€oll, Ch.; Kern, K. J. Phys. Chem. B<br />
2004, 108, 19392.<br />
(26) Ca~nas-Ventura, M. E.; Klappenberger, F.; Clair, S.; Pons, S.;<br />
Brune, H.; Kern, K.; Strunskus, T.; W€oll, C.; Fasel, R.; Barth, J. V.<br />
J. Chem. Phys. 2006, 125, No. 184710.<br />
(27) Dmitriev, A.; Spillmann, H.; Stepanow, S.; Strunskus, Th.;<br />
W€oll, Ch.; Seitsonen, A. P.; Lingenfelder, M.; Lin, N.; Barth, J. V.; Kern,<br />
K. Chem. Phys. Chem. 2006, 7, 2197.<br />
(28) O’Shea, J. N.; Schnadt, J.; Bru1hwiler, P. A.; Hillesheimer, H.;<br />
Martensson, N. J. Phys. Chem. B 2001, 105, 1917.<br />
(29) O’Shea, J. N.; Luo, Y.; Schnadt, J.; Pat<strong>the</strong>y, L.; Hillesheimer, H.;<br />
Krempasky, J.; Nordlund, D.; Nagasono, M.; Br€uhwiler, P. A.;<br />
Martensson, N. Surf. Sci. 2001, 486, 157.<br />
(30) Takusagawa, F.; Shimada, A. Acta Crystallogr. 1976, B32, 1925.<br />
(31) Schnadt, J.; O’Shea, J. N.; Pat<strong>the</strong>y, L.; Schiessling, J.;<br />
Krempasky, J.; Shi, M.; Martensson, N.; Br€uhwiler, P. A. Surf. Sci.<br />
2003, 544, 74.<br />
(32) Shi, Z.; Lin, N. J. Am. Chem. Soc. 2009, 131, 5376.<br />
(33) Katsonis, N.; Vicario, J.; Kudernac, T.; Visser, J.; Pollard, M. M.;<br />
Feringa, B. L. J. Am. Chem. Soc. 2006, 128, 15537.<br />
(34) Bai, Y.; Buchner, F.; Kellner, I.; Schmid, M.; Vollnhals, F.;<br />
Steinruck, H. P.; Marbach, H.; Gottfried, J. M. New J. Phys. 2009, 11, No.<br />
125004.<br />
(35) Dempsey, B., et al. In Haematin Enzymes; Falk, J. E., Lemberg,<br />
R., Morton, R. K., Eds.; Pergamon Press: New York, 1959; pp 29 37<br />
6854 dx.doi.org/10.1021/jp200533a |J. Phys. Chem. C 2011, 115, 6849–6854