Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1406 Chemical Reviews, 2000, Vol. 100, No. 4 Chen and Marks<br />
first example of alkyl group abstraction to yield the<br />
corresponding cationic metallocene species on a surface.<br />
Moreover, it served as a model <strong>for</strong> abstractive<br />
activation metallocene alkyls in solution, thus drawing<br />
direct connections between heterogeneous and<br />
homogeneous metallocene catalysis.<br />
1. Bis-Cp Type Group 4 <strong>Metal</strong>locene Activation<br />
The reaction of tris(pentafluorophenyl)borane,<br />
B(C6F5)3, with a variety of zirconocene dimethyl<br />
complexes proceeds rapidly and quantitatively at<br />
room temperature in noncoordinating solvents to<br />
yield cationic alkylzirconocene methyltriarylborate<br />
complexes (34; eq 26). 67 Reaction with excess of<br />
B(C6F5)3 does not effect the removal of the second<br />
metallocene methyl group, even after extended periods<br />
of reaction. 67a However, Green et al. 167 recently<br />
reported NMR spectroscopic evidence that a second<br />
1 equiv of FAB abstracts a second CH3 - group from<br />
the bis(benzyl-substituted Cp)zirconocene (p-MeC6H4-<br />
CMe2Cp)2ZrMe2 at -60 °C in CD2Cl2. Arene coordination<br />
of the Cp benzyl group to Zr is attributed to<br />
the stabilization of the resulting dication-like structure.<br />
However, this species reverts to the monocationic<br />
species and neutral FAB above -40 °C in<br />
solution.<br />
As noted above, the methyltris(pentafluorophenylborate)<br />
anion, MeB(C6F5)3 - , is a somewhat more<br />
coordinating anion with respect to metallocenium<br />
cations than the tetrakis borate anion, B(C6F5)4 - , and<br />
thus the relatively stronger cation-anion ion pairing<br />
stabilizes highly electron-deficient metal centers.<br />
This interaction improves hydrocarbon solubility,<br />
catalyst stability, and catalyst lifetime significantly.<br />
However, this coordination appears to be sufficiently<br />
weak and labile to allow an R-olefin to displace the<br />
anion from its coordination site <strong>for</strong> rapid enchainment.<br />
Unlike cations paired with B(C6F5)4 - , cationic<br />
complexes of the type Cp x 2Zr(CH3) + ---H3CB(C6F5)3 -<br />
are usually isolable and X-ray crystallographically<br />
characterizable. Because of these features, the versatility<br />
of FAB activation is advantageous. Thus, the<br />
catalyst precursor can be preactivated to yield an<br />
activated catalyst solution, can be activated in situ,<br />
and/or can be activated inside the reactor (in-reactor<br />
activation).<br />
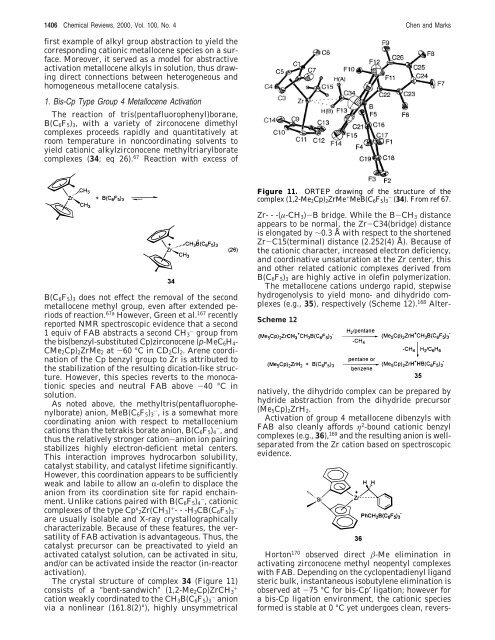
The crystal structure of complex 34 (Figure 11)<br />
consists of a “bent-sandwich” (1,2-Me2Cp)ZrCH3 +<br />
cation weakly coordinated to the CH3B(C6F5)3 - anion<br />
via a nonlinear (161.8(2)°), highly unsymmetrical<br />
Figure 11. ORTEP drawing of the structure of the<br />
complex (1,2-Me2Cp)2ZrMe + MeB(C6F5)3 - (34). From ref 67.<br />
Zr---(µ-CH3)-B bridge. While the B-CH3 distance<br />
appears to be normal, the Zr-C34(bridge) distance<br />
is elongated by ∼0.3 Å with respect to the shortened<br />
Zr-C15(terminal) distance (2.252(4) Å). Because of<br />
the cationic character, increased electron deficiency,<br />
and coordinative unsaturation at the Zr center, this<br />
and other related cationic complexes derived from<br />
B(C6F5)3 are highly active in olefin polymerization.<br />
The metallocene cations undergo rapid, stepwise<br />
hydrogenolysis to yield mono- and dihydrido complexes<br />
(e.g., 35), respectively (Scheme 12). 168 Alter-<br />
Scheme 12<br />
natively, the dihydrido complex can be prepared by<br />
hydride abstraction from the dihydride precursor<br />
(Me5Cp)2ZrH2.<br />
Activation of group 4 metallocene dibenzyls with<br />
FAB also cleanly af<strong>for</strong>ds η 2 -bound cationic benzyl<br />
complexes (e.g., 36), 169 and the resulting anion is wellseparated<br />
from the Zr cation based on spectroscopic<br />
evidence.<br />
Horton 170 observed direct �-Me elimination in<br />
activating zirconocene methyl neopentyl complexes<br />
with FAB. Depending on the cyclopentadienyl ligand<br />
steric bulk, instantaneous isobutylene elimination is<br />
observed at -75 °C <strong>for</strong> bis-Cp′ ligation; however <strong>for</strong><br />
a bis-Cp ligation environment, the cationic species<br />
<strong>for</strong>med is stable at 0 °C yet undergoes clean, revers-