Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1418 Chemical Reviews, 2000, Vol. 100, No. 4 Chen and Marks<br />
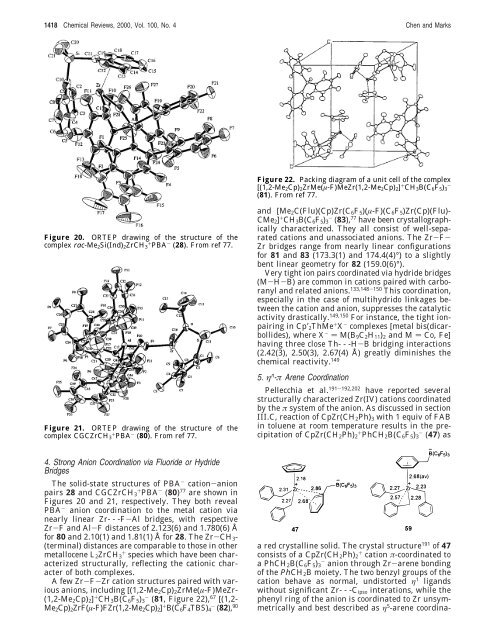
Figure 20. ORTEP drawing of the structure of the<br />
complex rac-Me2Si(Ind)2ZrCH3 + PBA - (28). From ref 77.<br />
Figure 21. ORTEP drawing of the structure of the<br />
complex CGCZrCH3 + PBA - (80). From ref 77.<br />
4. Strong Anion Coordination via Fluoride or Hydride<br />
Bridges<br />
The solid-state structures of PBA - cation-anion<br />
pairs 28 and CGCZrCH3 + PBA - (80) 77 are shown in<br />
Figures 20 and 21, respectively. They both reveal<br />
PBA - anion coordination to the metal cation via<br />
nearly linear Zr- - -F-Al bridges, with respective<br />
Zr-F and Al-F distances of 2.123(6) and 1.780(6) Å<br />
<strong>for</strong> 80 and 2.10(1) and 1.81(1) Å <strong>for</strong> 28. The Zr-CH3-<br />
(terminal) distances are comparable to those in other<br />
metallocene L2ZrCH3 + species which have been characterized<br />
structurally, reflecting the cationic character<br />
of both complexes.<br />
A few Zr-F-Zr cation structures paired with various<br />
anions, including [(1,2-Me2Cp)2ZrMe(µ-F)MeZr-<br />
(1,2-Me2Cp)2] + CH3B(C6F5)3 - (81, Figure 22), 67 [(1,2-<br />
Me2Cp)2ZrF(µ-F)FZr(1,2-Me2Cp)2] + B(C6F4TBS)4 - (82), 90<br />
Figure 22. Packing diagram of a unit cell of the complex<br />
[(1,2-Me2Cp)2ZrMe(µ-F)MeZr(1,2-Me2Cp)2] + CH3B(C6F5)3 -<br />
(81). From ref 77.<br />
and [Me2C(Flu)(Cp)Zr(C6F5)(µ-F)(C6F5)Zr(Cp)(Flu)-<br />
CMe2] + CH3B(C6F5)3 - (83), 77 have been crystallographically<br />
characterized. They all consist of well-separated<br />
cations and unassociated anions. The Zr-F-<br />
Zr bridges range from nearly linear configurations<br />
<strong>for</strong> 81 and 83 (173.3(1) and 174.4(4)°) to a slightly<br />
bent linear geometry <strong>for</strong> 82 (159.0(6)°).<br />
Very tight ion pairs coordinated via hydride bridges<br />
(M-H-B) are common in cations paired with carboranyl<br />
and related anions. 133,148-150 This coordination,<br />
especially in the case of multihydrido linkages between<br />
the cation and anion, suppresses the catalytic<br />
activity drastically. 149,150 For instance, the tight ionpairing<br />
in Cp′2ThMe + X - complexes [metal bis(dicarbollides),<br />
where X - ) M(B9C2H11)2 and M ) Co, Fe]<br />
having three close Th- - -H-B bridging interactions<br />
(2.42(3), 2.50(3), 2.67(4) Å) greatly diminishes the<br />
chemical reactivity. 149<br />
5. η n -π Arene Coordination<br />
Pellecchia et al. 191-192,202 have reported several<br />
structurally characterized Zr(IV) cations coordinated<br />
by the π system of the anion. As discussed in section<br />
III.C, reaction of CpZr(CH2Ph)3 with 1 equiv of FAB<br />
in toluene at room temperature results in the precipitation<br />
of CpZr(CH2Ph)2 + PhCH2B(C6F5)3 - (47) as<br />
a red crystalline solid. The crystal structure 191 of 47<br />
consists of a CpZr(CH2Ph)2 + cation π-coordinated to<br />
a PhCH2B(C6F5)3 - anion through Zr-arene bonding<br />
of the PhCH2B moiety. The two benzyl groups of the<br />
cation behave as normal, undistorted η 1 ligands<br />
without significant Zr- - -Cipso interations, while the<br />
phenyl ring of the anion is coordinated to Zr unsymmetrically<br />
and best described as η 5 -arene coordina-