Company Presentation January 2013 - Epigenomics AG
Company Presentation January 2013 - Epigenomics AG
Company Presentation January 2013 - Epigenomics AG
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
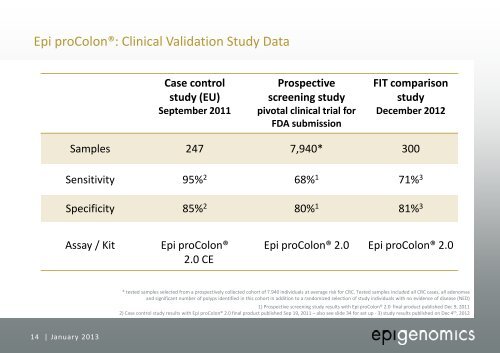
Epi proColon®: Clinical Validation Study Data<br />
14 | <strong>January</strong> <strong>2013</strong><br />
Case control<br />
study (EU)<br />
September 2011<br />
Prospective<br />
screening study<br />
pivotal clinical trial for<br />
FDA submission<br />
FIT comparison<br />
study<br />
December 2012<br />
Samples 247 7,940* 300<br />
Sensitivity 95% 2 68% 1 71% 3<br />
Specificity 85% 2 80% 1 81% 3<br />
Assay / Kit<br />
Epi proColon®<br />
2.0 CE<br />
Epi proColon® 2.0<br />
Epi proColon® 2.0<br />
* tested samples selected from a prospectively collected cohort of 7.940 individuals at average risk for CRC. Tested samples included all CRC cases, all adenomas<br />
and significant number of polyps identified in this cohort in addition to a randomized selection of study individuals with no evidence of disease (NED)<br />
1) Prospective screening study results with Epi proColon® 2.0 final product published Dec 9, 2011<br />
2) Case control study results with Epi proColon® 2.0 final product published Sep 19, 2011 – also see slide 34 for set up - 3) study results published on Dec 4 th , 2012