Link to Letter in Nature - Monash University
Link to Letter in Nature - Monash University
Link to Letter in Nature - Monash University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
RESEARCH LETTER<br />
a b<br />
c<br />
Y35<br />
P43<br />
F108<br />
Pre-Tα<br />
N<br />
C<br />
A192 S194 L149V147T145<br />
F58<br />
Y60<br />
Pre-Tα<br />
T71<br />
L73<br />
L32<br />
H75<br />
F131<br />
V28<br />
V30<br />
Cβ<br />
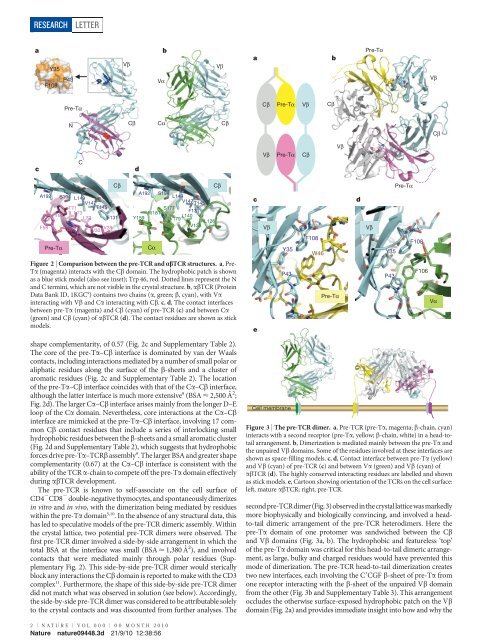
shape complementarity, of 0.57 (Fig. 2c and Supplementary Table 2).<br />
The core of the pre-Ta–Cb <strong>in</strong>terface is dom<strong>in</strong>ated by van der Waals<br />
contacts, <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>teractions mediated by a number of small polar or<br />
aliphatic residues along the surface of the b-sheets and a cluster of<br />
aromatic residues (Fig. 2c and Supplementary Table 2). The location<br />
of the pre-Ta–Cb <strong>in</strong>terface co<strong>in</strong>cides with that of the Ca–Cb <strong>in</strong>terface,<br />
although the latter <strong>in</strong>terface is much more extensive 9 (BSA < 2,500 A ˚ 2 ;<br />
Fig. 2d). The larger Ca–Cb <strong>in</strong>terface arises ma<strong>in</strong>ly from the longer D–E<br />
loop of the Ca doma<strong>in</strong>. Nevertheless, core <strong>in</strong>teractions at the Ca–Cb<br />
<strong>in</strong>terface are mimicked at the pre-Ta–Cb <strong>in</strong>terface, <strong>in</strong>volv<strong>in</strong>g 17 common<br />
Cb contact residues that <strong>in</strong>clude a series of <strong>in</strong>terlock<strong>in</strong>g small<br />
hydrophobic residues between the b-sheets and a small aromatic cluster<br />
(Fig. 2d and Supplementary Table 2), which suggests that hydrophobic<br />
forces drive pre-Ta–TCRb assembly 9 . The larger BSA and greater shape<br />
complementarity (0.67) at the Ca–Cb <strong>in</strong>terface is consistent with the<br />
ability of the TCR a-cha<strong>in</strong> <strong>to</strong> compete off the pre-Ta doma<strong>in</strong> effectively<br />
dur<strong>in</strong>g abTCR development.<br />
The pre-TCR is known <strong>to</strong> self-associate on the cell surface of<br />
CD4 2 CD8 2 double-negative thymocytes, and spontaneously dimerizes<br />
<strong>in</strong> vitro and <strong>in</strong> vivo, with the dimerization be<strong>in</strong>g mediated by residues<br />
with<strong>in</strong> the pre-Ta doma<strong>in</strong> 5,10 . In the absence of any structural data, this<br />
has led <strong>to</strong> speculative models of the pre-TCR dimeric assembly. With<strong>in</strong><br />
the crystal lattice, two potential pre-TCR dimers were observed. The<br />
first pre-TCR dimer <strong>in</strong>volved a side-by-side arrangement <strong>in</strong> which the<br />
<strong>to</strong>tal BSA at the <strong>in</strong>terface was small (BSA < 1,380 A ˚ 2 ), and <strong>in</strong>volved<br />
contacts that were mediated ma<strong>in</strong>ly through polar residues (Supplementary<br />
Fig. 2). This side-by-side pre-TCR dimer would sterically<br />
block any <strong>in</strong>teractions the Cb doma<strong>in</strong> is reported <strong>to</strong> make with the CD3<br />
complex 11 . Furthermore, the shape of this side-by-side pre-TCR dimer<br />
did not match what was observed <strong>in</strong> solution (see below). Accord<strong>in</strong>gly,<br />
the side-by-side pre-TCR dimer was considered <strong>to</strong> be attributable solely<br />
<strong>to</strong> the crystal contacts and was discounted from further analyses. The<br />
2 | NATURE | VOL 000 | 00 MONTH 2010<br />
<strong>Nature</strong> nature09448.3d 21/9/10 12:38:56<br />
Vβ<br />
Cβ<br />
Vα<br />
Cα<br />
A192 S194 L149V147T145<br />
F131<br />
Y159 W181<br />
S177<br />
L140<br />
V179<br />
V138 L128<br />
Figure 2 | Comparison between the pre-TCR and abTCR structures. a, Pre-<br />
Ta (magenta) <strong>in</strong>teracts with the Cb doma<strong>in</strong>. The hydrophobic patch is shown<br />
as a blue stick model (also see <strong>in</strong>set); Trp 46, red. Dotted l<strong>in</strong>es represent the N<br />
and C term<strong>in</strong>i, which are not visible <strong>in</strong> the crystal structure. b, abTCR (Prote<strong>in</strong><br />
Data Bank ID, 1KGC 9 ) conta<strong>in</strong>s two cha<strong>in</strong>s (a, green; b, cyan), with Va<br />
<strong>in</strong>teract<strong>in</strong>g with Vb and Ca <strong>in</strong>teract<strong>in</strong>g with Cb. c, d, The contact <strong>in</strong>terfaces<br />
between pre-Ta (magenta) and Cb (cyan) of pre-TCR (c) and between Ca<br />
(green) and Cb (cyan) of abTCR (d). The contact residues are shown as stick<br />
models.<br />
d<br />
Cα<br />
Vβ<br />
Cβ<br />
Cβ<br />
a b<br />
c<br />
e<br />
Cβ<br />
Vβ<br />
Vβ<br />
Pre-Tα<br />
Pre-Tα<br />
Y35<br />
P43<br />
Cell membrane<br />
Vβ<br />
Cβ<br />
F108<br />
W46<br />
Cβ<br />
Pre-Tα<br />
Vβ<br />
Pre-Tα<br />
Pre-Tα<br />
secondpre-TCRdimer(Fig.3)observed<strong>in</strong>thecrystallatticewasmarkedly<br />
more biophysically and biologically conv<strong>in</strong>c<strong>in</strong>g, and <strong>in</strong>volved a head<strong>to</strong>-tail<br />
dimeric arrangement of the pre-TCR heterodimers. Here the<br />
pre-Ta doma<strong>in</strong> of one pro<strong>to</strong>mer was sandwiched between the Cb<br />
and Vb doma<strong>in</strong>s (Fig. 3a, b). The hydrophobic and featureless ‘<strong>to</strong>p’<br />
of the pre-Ta doma<strong>in</strong> was critical for this head-<strong>to</strong>-tail dimeric arrangement,<br />
as large, bulky and charged residues would have prevented this<br />
mode of dimerization. The pre-TCR head-<strong>to</strong>-tail dimerization creates<br />
two new <strong>in</strong>terfaces, each <strong>in</strong>volv<strong>in</strong>g the C9CGF b-sheet of pre-Ta from<br />
one recep<strong>to</strong>r <strong>in</strong>teract<strong>in</strong>g with the b-sheet of the unpaired Vb doma<strong>in</strong><br />
from the other (Fig. 3b and Supplementary Table 3). This arrangement<br />
occludes the otherwise surface-exposed hydrophobic patch on the Vb<br />
doma<strong>in</strong> (Fig. 2a) and provides immediate <strong>in</strong>sight <strong>in</strong><strong>to</strong> how and why the<br />
d<br />
Vβ<br />
Y35<br />
P43<br />
F108<br />
F106<br />
Figure 3 | The pre-TCR dimer. a, Pre-TCR (pre-Ta, magenta; b-cha<strong>in</strong>, cyan)<br />
<strong>in</strong>teracts with a second recep<strong>to</strong>r (pre-Ta, yellow; b-cha<strong>in</strong>, white) <strong>in</strong> a head-<strong>to</strong>tail<br />
arrangement. b, Dimerization is mediated ma<strong>in</strong>ly between the pre-Ta and<br />
the unpaired Vb doma<strong>in</strong>s. Some of the residues <strong>in</strong>volved at these <strong>in</strong>terfaces are<br />
shown as space-fill<strong>in</strong>g models. c, d, Contact <strong>in</strong>terface between pre-Ta (yellow)<br />
and Vb (cyan) of pre-TCR (c) and between Va (green) and Vb (cyan) of<br />
abTCR (d). The highly conserved <strong>in</strong>teract<strong>in</strong>g residues are labelled and shown<br />
as stick models. e, Car<strong>to</strong>on show<strong>in</strong>g orientation of the TCRs on the cell surface:<br />
left, mature abTCR; right, pre-TCR.<br />
Vβ<br />
Cβ<br />
Vα