4.3.2. Identifikasi Kuantitatif dan Kualitatif Fasa BaFe Co Zn O
4.3.2. Identifikasi Kuantitatif dan Kualitatif Fasa BaFe Co Zn O
4.3.2. Identifikasi Kuantitatif dan Kualitatif Fasa BaFe Co Zn O
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
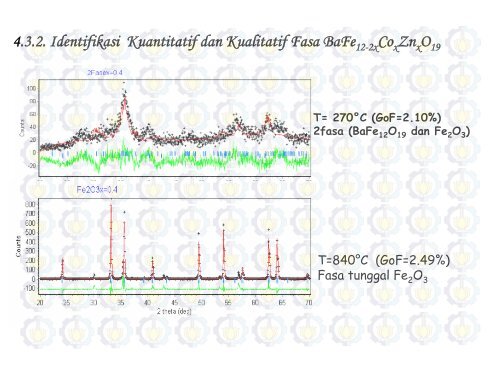
<strong>4.3.2.</strong> <strong>Identifikasi</strong> <strong>Kuantitatif</strong> <strong>dan</strong> <strong>Kualitatif</strong> <strong>Fasa</strong> <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 T= 270°C (GoF=2.10%)<br />
2fasa (<strong>BaFe</strong>12O19 <strong>dan</strong><br />
Fe2O3 T=840°C (GoF=2.49%)<br />
<strong>Fasa</strong> tunggal<br />
Fe2O3 )
<strong>4.3.2.</strong> <strong>Identifikasi</strong> <strong>Kuantitatif</strong> <strong>dan</strong> <strong>Kualitatif</strong> <strong>Fasa</strong> <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 Intensitas<br />
M<br />
1200°Cα<br />
M<br />
1000°C<br />
900°C<br />
840°C<br />
700°C<br />
270°C<br />
200°C<br />
80°C<br />
20 25 30 35 40 45 50 55 60 65 70<br />
Tipe-M<br />
α-Fe2O3 Tipe-W<br />
M<br />
W α M<br />
W α M<br />
M<br />
α M α α M<br />
W M α<br />
W αMα 20 25 30 35 40 45 50 55 60 65 70<br />
Pola difraksi XRD precursor <strong>BaFe</strong> 11.2 <strong>Co</strong> 0.4 <strong>Zn</strong> 0.4 O 19 yang di sintering pada berbagai temperatur<br />
α
<strong>4.3.2.</strong> <strong>Identifikasi</strong> <strong>Kuantitatif</strong> <strong>dan</strong> <strong>Kualitatif</strong> <strong>Fasa</strong> <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 Keluaran hasil refenement Maud<br />
T= 840°C (Sig=1.37%)<br />
pada precursor <strong>BaFe</strong>10.4<strong>Co</strong>0.8<strong>Zn</strong>0.8O19
4.4 Analisis Mikrostruktur Barium M-Hexaferrit<br />
M Hexaferrit <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19)<br />
<strong>BaFe</strong>12 2x<strong>Co</strong>x<strong>Zn</strong>xO19)<br />
(a)<br />
Foto TEM prekursor <strong>BaFe</strong> 12-2x <strong>Co</strong> x <strong>Zn</strong> x O 19 pada (a) x=0.4 T=270°C (b) x=0.6 T=840°C,<br />
(c) x=0.6 T=900°C <strong>dan</strong> (d) x=0.4 T=1200°C.<br />
(b)<br />
(c) (d)
4.4 Analisis Mikrostruktur Barium M-Hexaferrit<br />
M Hexaferrit <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19)<br />
<strong>BaFe</strong>12 2x<strong>Co</strong>x<strong>Zn</strong>xO19)<br />
(a) (b)<br />
Foto SEM prekursor <strong>BaFe</strong> 12-2x <strong>Co</strong> x <strong>Zn</strong> x O 19 pada komposisi substitusi (a) x=0.2, (b) x=0.6, <strong>dan</strong> (c) x=0.8<br />
(c)
4.5 Interprestasi Proses Substitusi Ion Dopan <strong>Co</strong>/<strong>Zn</strong> terhadap<br />
Struktur Kristal (<strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19) Tabel Puncak transmisi FTIR (1/λ=cm-1 ) precursor <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 T=80°C 2 jam<br />
x=0.6<br />
421 (s)<br />
687 (s)<br />
841 (m)<br />
1080(w)<br />
1408(w)<br />
1628(m)<br />
3383(s)<br />
T=270°C 4 jam T=840°C 4 jam<br />
x=0 x=0.4 x=1<br />
482(m )<br />
574 (s)<br />
1084(w)<br />
1385(w)<br />
1647(w)<br />
3422(w)<br />
3448(w)<br />
421 (s)<br />
436 (s)<br />
575 (w)<br />
1080(w)<br />
1408(w)<br />
1628(m)<br />
3422(s)<br />
421(s)<br />
575(s)<br />
849(m)<br />
1088(m)<br />
1254(w)<br />
1408(w)<br />
1628(m)<br />
3387(s)<br />
3421(s)<br />
x=0.4<br />
478 (s)<br />
575 (s)<br />
1076 (w)<br />
1520 (w)<br />
1640 (w)<br />
3449 (w)<br />
T=900°C 4 jam<br />
x=0.6<br />
478 (s)<br />
552 (s)<br />
575 (s)<br />
3422(w)
1.<br />
2.<br />
3.<br />
4.<br />
5.<br />
Kesimpulan<br />
Sintesis barium M-hexaferrit <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 dengan metode<br />
kopresipitasi mempunyai konsentrasi yang tinggi pada substitusi<br />
x=0.4.<br />
Berdasarkan analisis DTA/TGA <strong>dan</strong> XRD, proses sentering pada<br />
T≥740°C cenderung membentuk fasa hematite (α-Fe2O3) Proses sintesis barium M-hexaferrit <strong>BaFe</strong>12-2x<strong>Co</strong>x<strong>Zn</strong>xO19 dengan<br />
metode kopresipitasi mempunyai rata-rata orde partikel 50 nm.<br />
Pendopingan ion dopan <strong>Co</strong>/<strong>Zn</strong> pada sintesis barium M-hexaferrit<br />
tidak merubah struktur dasar kristal (hexagonal).<br />
Peningkatan variabel ion dopan <strong>Co</strong>/<strong>Zn</strong> akan menyebabkan<br />
peningkatan ukuran partikel prekursor barium M-hexaferrit.