T.Mazzei, A.Novelli pdf - Sipps
T.Mazzei, A.Novelli pdf - Sipps
T.Mazzei, A.Novelli pdf - Sipps
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
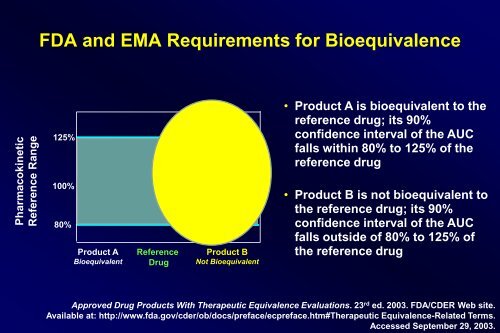
Pharmacokinetic<br />
Reference Range<br />
FDA and EMA Requirements for Bioequivalence<br />
125%<br />
100%<br />
80%<br />
Product A<br />
Bioequivalent<br />
Reference<br />
Drug<br />
Product B<br />
Not Bioequivalent<br />
• Product A is bioequivalent to the<br />
reference drug; its 90%<br />
confidence interval of the AUC<br />
falls within 80% to 125% of the<br />
reference drug<br />
• Product B is not bioequivalent to<br />
the reference drug; its 90%<br />
confidence interval of the AUC<br />
falls outside of 80% to 125% of<br />
the reference drug<br />
Approved Drug Products With Therapeutic Equivalence Evaluations. 23 rd ed. 2003. FDA/CDER Web site.<br />
Available at: http://www.fda.gov/cder/ob/docs/preface/ecpreface.htm#Therapeutic Equivalence-Related Terms.<br />
Accessed September 29, 2003.