Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
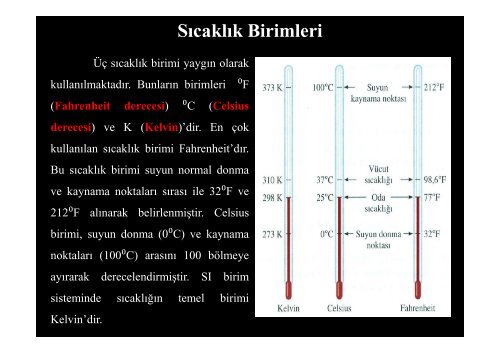
Üç s<strong>ı</strong>cakl<strong>ı</strong>k birimi yayg<strong>ı</strong>n olarak<br />
kullan<strong>ı</strong>lmaktad<strong>ı</strong>r. Bunlar<strong>ı</strong>n birimleri<br />
S<strong>ı</strong>cakl<strong>ı</strong>k Birimleri<br />
⁰F<br />
(Fahrenheit derecesi) ⁰C (Celsius<br />
derecesi) ve K (Kelvin)’dir. En çok<br />
kullan<strong>ı</strong>lan s<strong>ı</strong>cakl<strong>ı</strong>k birimi Fahrenheit’d<strong>ı</strong>r.<br />
Bu s<strong>ı</strong>cakl<strong>ı</strong>k birimi suyun normal donma<br />
ve kaynama noktalar<strong>ı</strong> s<strong>ı</strong>ras<strong>ı</strong> ile 32⁰F⁰<br />
ve<br />
212⁰F al<strong>ı</strong>narak belirlenmiştir. Celsius<br />
birimi, suyun donma (0⁰C) ve kaynama<br />
noktalar<strong>ı</strong> (100⁰C) aras<strong>ı</strong>n<strong>ı</strong> 100 bölmeye<br />
ay<strong>ı</strong>rarak derecelendirmiştir. SI birim<br />
sisteminde s<strong>ı</strong>cakl<strong>ı</strong>ğ<strong>ı</strong>n temel birimi<br />
Kelvin’dir.