Silber spiegel - SwissEduc
Silber spiegel - SwissEduc
Silber spiegel - SwissEduc
Sie wollen auch ein ePaper? Erhöhen Sie die Reichweite Ihrer Titel.
YUMPU macht aus Druck-PDFs automatisch weboptimierte ePaper, die Google liebt.
© c+b 3/04<br />
Mechanisms<br />
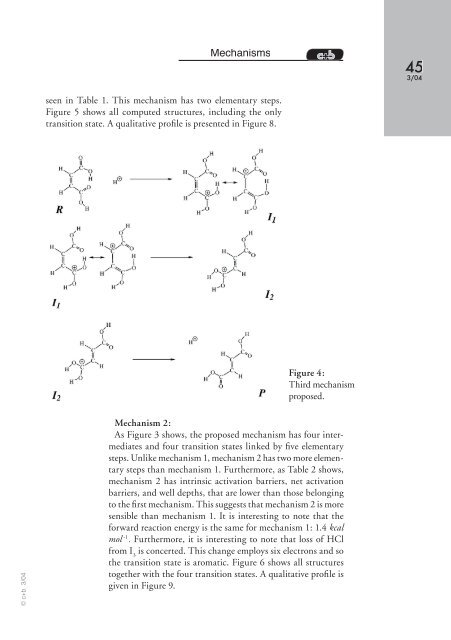
seen in Table 1. This mechanism has two elementary steps.<br />
Figure 5 shows all computed structures, including the only<br />
transition state. A qualitative profi le is presented in Figure 8.<br />
Figure 4:<br />
Third mechanism<br />
proposed.<br />
Mechanism 2:<br />
As Figure 3 shows, the proposed mechanism has four intermediates<br />
and four transition states linked by fi ve elementary<br />
steps. Unlike mechanism 1, mechanism 2 has two more elementary<br />
steps than mechanism 1. Furthermore, as Table 2 shows,<br />
mechanism 2 has intrinsic activation barriers, net activation<br />
barriers, and well depths, that are lower than those belonging<br />
to the fi rst mechanism. This suggests that mechanism 2 is more<br />
sensible than mechanism 1. It is interesting to note that the<br />
forward reaction energy is the same for mechanism 1: 1.4 kcal<br />
mol -1 Fig.4<br />
. Furthermore, it is interesting to note that loss of HCl<br />
from I is concerted. This change employs six electrons and so<br />
3<br />
the transition state is aromatic. Figure 6 shows all structures<br />
together with the four transition states. A qualitative profi le is<br />
given in Figure 9.<br />
45<br />
3/04


![PDF [760 KB] - SwissEduc.ch](https://img.yumpu.com/23380241/1/184x260/pdf-760-kb-swisseducch.jpg?quality=85)

![Klimafaktoren Nordamerika - PDF [544 KB] - SwissEduc.ch](https://img.yumpu.com/21981097/1/184x260/klimafaktoren-nordamerika-pdf-544-kb-swisseducch.jpg?quality=85)





![PDF [522 KB] - SwissEduc.ch](https://img.yumpu.com/21660668/1/184x260/pdf-522-kb-swisseducch.jpg?quality=85)
![PDF [88 KB] - SwissEduc.ch](https://img.yumpu.com/21573927/1/184x260/pdf-88-kb-swisseducch.jpg?quality=85)
![PDF [36 MB] - SwissEduc.ch](https://img.yumpu.com/20710777/1/190x135/pdf-36-mb-swisseducch.jpg?quality=85)