Silber spiegel - SwissEduc
Silber spiegel - SwissEduc
Silber spiegel - SwissEduc
Sie wollen auch ein ePaper? Erhöhen Sie die Reichweite Ihrer Titel.
YUMPU macht aus Druck-PDFs automatisch weboptimierte ePaper, die Google liebt.
46<br />
3/04<br />
Mechanisms<br />
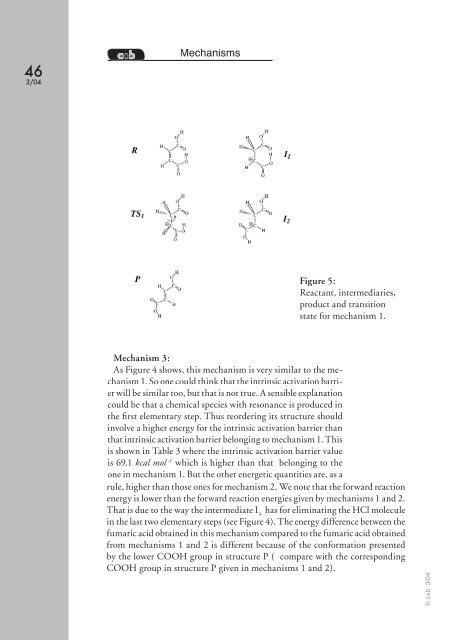
Figure 5:<br />
Reactant, intermediaries,<br />
product and transition<br />
state for mechanism 1.<br />
Fig.5<br />
Mechanism 3:<br />
As Figure 4 shows, this mechanism is very similar to the mechanism<br />
1. So one could think that the intrinsic activation barrier<br />
will be similar too, but that is not true. A sensible explanation<br />
could be that a chemical species with resonance is produced in<br />
the fi rst elementary step. Thus reordering its structure should<br />
involve a higher energy for the intrinsic activation barrier than<br />
that intrinsic activation barrier belonging to mechanism 1. This<br />
is shown in Table 3 where the intrinsic activation barrier value<br />
is 69.1 kcal mol -1 which is higher than that belonging to the<br />
one in mechanism 1. But the other energetic quantities are, as a<br />
rule, higher than those ones for mechanism 2. We note that the forward reaction<br />
energy is lower than the forward reaction energies given by mechanisms 1 and 2.<br />
That is due to the way the intermediate I 3 has for eliminating the HCl molecule<br />
in the last two elementary steps (see Figure 4). The energy difference between the<br />
fumaric acid obtained in this mechanism compared to the fumaric acid obtained<br />
from mechanisms 1 and 2 is different because of the conformation presented<br />
by the lower COOH group in structure P ( compare with the corresponding<br />
COOH group in structure P given in mechanisms 1 and 2).<br />
© c+b 3/04


![PDF [760 KB] - SwissEduc.ch](https://img.yumpu.com/23380241/1/184x260/pdf-760-kb-swisseducch.jpg?quality=85)

![Klimafaktoren Nordamerika - PDF [544 KB] - SwissEduc.ch](https://img.yumpu.com/21981097/1/184x260/klimafaktoren-nordamerika-pdf-544-kb-swisseducch.jpg?quality=85)





![PDF [522 KB] - SwissEduc.ch](https://img.yumpu.com/21660668/1/184x260/pdf-522-kb-swisseducch.jpg?quality=85)
![PDF [88 KB] - SwissEduc.ch](https://img.yumpu.com/21573927/1/184x260/pdf-88-kb-swisseducch.jpg?quality=85)
![PDF [36 MB] - SwissEduc.ch](https://img.yumpu.com/20710777/1/190x135/pdf-36-mb-swisseducch.jpg?quality=85)


![PDF [51 KB] - SwissEduc.ch](https://img.yumpu.com/20710759/1/184x260/pdf-51-kb-swisseducch.jpg?quality=85)