Enterprise - Medical Devices - List of Competent Authorities
Enterprise - Medical Devices - List of Competent Authorities
Enterprise - Medical Devices - List of Competent Authorities
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
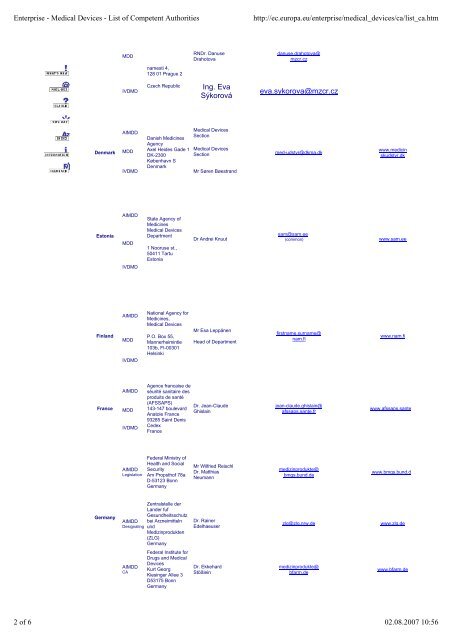
Denmark<br />
Estonia<br />
Finland<br />
France<br />
Germany<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
namesti 4,<br />
128 01 Prague 2<br />
Czech Republic<br />
Danish Medicines<br />
Agency<br />
Axel Heides Gade 1<br />
DK-2300<br />
København S<br />
RNDr. Danuse<br />
Drahotova<br />
Ing. Eva<br />
Sýkorová<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Section<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Section<br />
Denmark<br />
IVDMD Mr Søren Bøestrand<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
Legislation<br />
State Agency <strong>of</strong><br />
Medicines<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Department<br />
1 Nooruse st.,<br />
50411 Tartu<br />
Estonia<br />
National Agency for<br />
Medicines,<br />
<strong>Medical</strong> <strong>Devices</strong><br />
P.O. Box 55,<br />
Mannerheimintie<br />
103b, FI-00301<br />
Helsinki<br />
Agence francaise de<br />
séurité sanitaire des<br />
produits de santé<br />
(AFSSAPS)<br />
143-147 boulevard<br />
Anatole France<br />
93285 Saint Denis<br />
Cedex<br />
France<br />
Federal Ministry <strong>of</strong><br />
Health and Social<br />
Security<br />
Am Propsth<strong>of</strong> 78a<br />
D-53123 Bonn<br />
Germany<br />
Zentralstelle der<br />
Lander fuf<br />
Gesundheitsschutz<br />
AIMDD bei Arzneimitteln<br />
Designating und<br />
Medizinprodukten<br />
(ZLG)<br />
Germany<br />
AIMDD<br />
CA<br />
Federal Institute for<br />
Drugs and <strong>Medical</strong><br />
<strong>Devices</strong><br />
Kurt Georg<br />
Kiesinger Allee 3<br />
D53175 Bonn<br />
Germany<br />
Dr Andrei Knuut<br />
Mr Esa Leppänen<br />
Head <strong>of</strong> Department<br />
Dr. Jean-Claude<br />
Ghislain<br />
Mr Wilfried Reischl<br />
Dr. Matthias<br />
Neumann<br />
Dr. Rainer<br />
Edelhaeuser<br />
Dr. Ekkehard<br />
Stößlein<br />
danuse.drahotova@<br />
mzcr.cz<br />
eva.sykorova@mzcr.cz<br />
med-udstyr@dkma.dk<br />
sam@sam.ee<br />
(common)<br />
firstname.surname@<br />
nam.fi<br />
jean-claude.ghislain@<br />
afssaps.sante.fr<br />
medizinprodukte@<br />
bmgs.bund.de<br />
www.medicin<br />
skudstyr.dk<br />
www.sam.ee<br />
www.nam.fi<br />
www.afssaps.sante.fr<br />
www.bmgs.bund.de<br />
zlg@zlg.nrw.de www.zlg.de<br />
medizinprodukte@<br />
bfarm.de<br />
www.bfarm.de<br />
2 <strong>of</strong> 6 02.08.2007 10:56