Enterprise - Medical Devices - List of Competent Authorities
Enterprise - Medical Devices - List of Competent Authorities
Enterprise - Medical Devices - List of Competent Authorities
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
IMPORTANT LEGAL NOTICE - The information on this site is subject to a disclaimer and a copyright notice.<br />
Home Industry Sectors <strong>Medical</strong> devices<br />
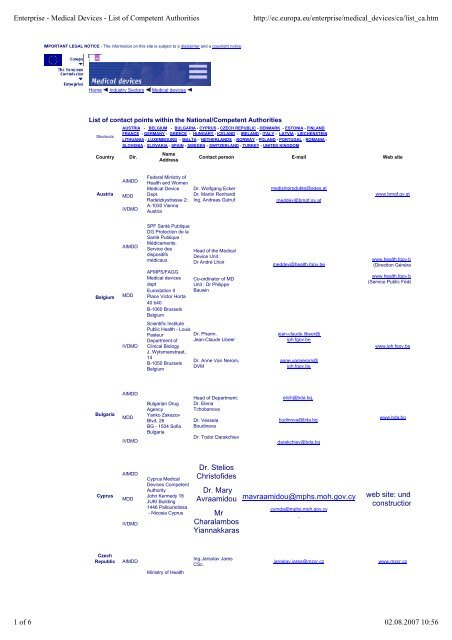
<strong>List</strong> <strong>of</strong> contact points within the National/<strong>Competent</strong> <strong>Authorities</strong><br />
Shortcuts<br />
Country Dir.<br />
Austria<br />
Belgium<br />
Bulgaria<br />
Cyprus<br />
Czech<br />
Republic<br />
AUSTRIA - BELGIUM - BULGARIA - CYPRUS - CZECH REPUBLIC - DENMARK - ESTONIA - FINLAND<br />
FRANCE - GERMANY - GREECE - HUNGARY - ICELAND - IRELAND - ITALY - LATVIA - LIECHENSTEIN<br />
LITHUANIA - LUXEMBOURG - MALTA - NETHERLANDS - NORWAY - POLAND - PORTUGAL - ROMANIA -<br />
SLOVENIA - SLOVAKIA - SPAIN - SWEDEN - SWITZERLAND - TURKEY - UNITED KINGDOM<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
Name<br />
Address<br />
Federal Ministry <strong>of</strong><br />
Health and Women<br />
<strong>Medical</strong> Device<br />
Dept.<br />
Radetzkystrasse 2;<br />
A-1030 Vienna<br />
Austria<br />
SPF Santè Publique<br />
DG Protection de la<br />
Santè Publique :<br />
Médicaments.<br />
Service des<br />
dispositifs<br />
médicaux.<br />
AFMPS/FAGG<br />
<strong>Medical</strong> devices<br />
dept<br />
Eurostation II<br />
Place Victor Horta<br />
40 b40<br />
B-1060 Brussels<br />
Belgium<br />
Scientific Institute<br />
Public Health - Louis<br />
Pasteur<br />
Department <strong>of</strong><br />
Clinical Biology<br />
J. Wytsmanstraat,<br />
14<br />
B-1050 Brussels<br />
Belgium<br />
Bulgarian Drug<br />
Agency<br />
Yanko Zakazov<br />
Blvd, 26<br />
BG - 1504 S<strong>of</strong>ia<br />
Bulgaria<br />
Cyprus <strong>Medical</strong><br />
<strong>Devices</strong> <strong>Competent</strong><br />
Authority<br />
John Kennedy 18<br />
JUKI Building<br />
1446 Pallouriotissa<br />
- Nicosia Cyprus<br />
Ministry <strong>of</strong> Health<br />
Contact person E-mail Web site<br />
Dr. Wolfgang Ecker<br />
Dr. Martin Renhardt<br />
Ing. Andreas Gutruf<br />
Head <strong>of</strong> the <strong>Medical</strong><br />
Device Unit :<br />
Dr André Lhoir<br />
Co-ordinator <strong>of</strong> MD<br />
Unit : Dr Philippe<br />
Bauwin<br />
Dr. Pharm.<br />
Jean-Claude Libeer<br />
Dr. Anne Van Nerom,<br />
DVM<br />
Head <strong>of</strong> Department:<br />
Dr. Elena<br />
Tchobanova<br />
Dr. Vessela<br />
Boudinova<br />
Dr. Todor Darakchiev<br />
Dr. Stelios<br />
Christ<strong>of</strong>ides<br />
Dr. Mary<br />
Avraamidou<br />
Mr<br />
Charalambos<br />
Yiannakkaras<br />
Ing.Jarsolav Jares<br />
CSc.<br />
medizinprodukte@ages.at<br />
meddev@bmgf.gv.at<br />
meddev@health.fgov.be<br />
jean-claude.libeer@<br />
iph.fgov.be<br />
anne.vannerom@<br />
iph.fgov.be<br />
elich@bda.bg,<br />
budinova@bda.bg<br />
darakchiev@bda.bg<br />
mavraamidou@mphs.moh.gov.cy<br />
cymda@mphs.moh.gov.cy<br />
www.bmgf.gv.at<br />
www.health.fgov.be<br />
(Direction Général)<br />
www.health.fgov.be<br />
(Service Public Fédéral)<br />
www.iph.fgov.be<br />
www.bda.bg<br />
web site: under<br />
construction<br />
jaroslav.jares@mzcr.cz www.mzcr.cz<br />
1 <strong>of</strong> 6 02.08.2007 10:56
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
Denmark<br />
Estonia<br />
Finland<br />
France<br />
Germany<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
namesti 4,<br />
128 01 Prague 2<br />
Czech Republic<br />
Danish Medicines<br />
Agency<br />
Axel Heides Gade 1<br />
DK-2300<br />
København S<br />
RNDr. Danuse<br />
Drahotova<br />
Ing. Eva<br />
Sýkorová<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Section<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Section<br />
Denmark<br />
IVDMD Mr Søren Bøestrand<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
Legislation<br />
State Agency <strong>of</strong><br />
Medicines<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Department<br />
1 Nooruse st.,<br />
50411 Tartu<br />
Estonia<br />
National Agency for<br />
Medicines,<br />
<strong>Medical</strong> <strong>Devices</strong><br />
P.O. Box 55,<br />
Mannerheimintie<br />
103b, FI-00301<br />
Helsinki<br />
Agence francaise de<br />
séurité sanitaire des<br />
produits de santé<br />
(AFSSAPS)<br />
143-147 boulevard<br />
Anatole France<br />
93285 Saint Denis<br />
Cedex<br />
France<br />
Federal Ministry <strong>of</strong><br />
Health and Social<br />
Security<br />
Am Propsth<strong>of</strong> 78a<br />
D-53123 Bonn<br />
Germany<br />
Zentralstelle der<br />
Lander fuf<br />
Gesundheitsschutz<br />
AIMDD bei Arzneimitteln<br />
Designating und<br />
Medizinprodukten<br />
(ZLG)<br />
Germany<br />
AIMDD<br />
CA<br />
Federal Institute for<br />
Drugs and <strong>Medical</strong><br />
<strong>Devices</strong><br />
Kurt Georg<br />
Kiesinger Allee 3<br />
D53175 Bonn<br />
Germany<br />
Dr Andrei Knuut<br />
Mr Esa Leppänen<br />
Head <strong>of</strong> Department<br />
Dr. Jean-Claude<br />
Ghislain<br />
Mr Wilfried Reischl<br />
Dr. Matthias<br />
Neumann<br />
Dr. Rainer<br />
Edelhaeuser<br />
Dr. Ekkehard<br />
Stößlein<br />
danuse.drahotova@<br />
mzcr.cz<br />
eva.sykorova@mzcr.cz<br />
med-udstyr@dkma.dk<br />
sam@sam.ee<br />
(common)<br />
firstname.surname@<br />
nam.fi<br />
jean-claude.ghislain@<br />
afssaps.sante.fr<br />
medizinprodukte@<br />
bmgs.bund.de<br />
www.medicin<br />
skudstyr.dk<br />
www.sam.ee<br />
www.nam.fi<br />
www.afssaps.sante.fr<br />
www.bmgs.bund.de<br />
zlg@zlg.nrw.de www.zlg.de<br />
medizinprodukte@<br />
bfarm.de<br />
www.bfarm.de<br />
2 <strong>of</strong> 6 02.08.2007 10:56
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
MDD<br />
Legislation<br />
Federal Ministry <strong>of</strong><br />
Health and Social<br />
Security<br />
Am Propsth<strong>of</strong> 78a<br />
D-53123 Bonn<br />
Germany<br />
Zentralstelle der<br />
Lander fur<br />
Gesundheitsschutz<br />
MDD bei Arzneimitteln<br />
Designating und<br />
Medizinprodukten<br />
(ZLG)<br />
Germany<br />
MDD<br />
CA<br />
IVDMD<br />
Legislation<br />
Federal Institute for<br />
Drugs and <strong>Medical</strong><br />
<strong>Devices</strong><br />
Kurt Georg<br />
Kiesinger Allee 3<br />
D53175 Bonn<br />
Germany<br />
Federal Ministry <strong>of</strong><br />
Health and Social<br />
Security<br />
Am Propsth<strong>of</strong> 78a<br />
D-53123 Bonn<br />
Germany<br />
Zentralstelle der<br />
Lander fur<br />
Gesundheitsschutz<br />
IVDMD bei Arzneimitteln<br />
Designating und<br />
Medizinprodukten<br />
(ZLG)<br />
Germany<br />
IVDMD 1<br />
CA<br />
IVDMD 2<br />
CA<br />
Federal Institute for<br />
Drugs and <strong>Medical</strong><br />
<strong>Devices</strong><br />
Kurt Georg<br />
Kiesinger Allee 3<br />
D53175 Bonn<br />
Mr Wilfried Reischl<br />
Dr. Matthias<br />
Neumann<br />
Dr. Rainer<br />
Edelhaeuser<br />
Dr. Ekkehard<br />
Stößlein<br />
Mr Wilfried Reischl<br />
Dr. Matthias<br />
Neumann<br />
Dr. Rainer<br />
Edelhaeuser<br />
Dr. Rüdiger<br />
Siekmeier<br />
medizinprodukte@<br />
bmg.bund.de<br />
www.bmgs.<br />
bund.de<br />
zlg@zlg.nrw.de www.zlg.de<br />
medizinprodukte@<br />
bfarm.de<br />
medizinprodukte@<br />
bmg.bund.de<br />
www.bfarm.de<br />
www.bmgs.<br />
bund.de<br />
zlg@zlg.nrw.de www.zlg.de<br />
medizinprodukte@<br />
bfarm.de<br />
www.bfarm.de<br />
Paul Ehrlich Institute<br />
Paul-Ehrlich-Strasse<br />
51-59<br />
D 63225 Langen<br />
Germany<br />
Jochen Halbauer s-ivd@pei.de www.pei.de<br />
1 ex those Annex II devices<br />
2 Annex II products as far as related to infection diseases or immuno haematology or tissue typing<br />
Greece<br />
Hungary<br />
Iceland<br />
(EFTA)<br />
Ireland<br />
AIMDD<br />
MDD<br />
IVDMD<br />
National<br />
Organization for<br />
Medicines<br />
284 Mesogion Ave<br />
155 62<br />
Holargos-Athens<br />
Greece<br />
Mrs Vasiliki Revithi vrevithi@e<strong>of</strong>.gr www.e<strong>of</strong>.gr<br />
AIMDD Authority for <strong>Medical</strong><br />
<strong>Devices</strong><br />
MDD Budapest 1245 Mr Peter Bunyitai amd@eekh.hu http://www.eekh.hu<br />
IVDMD<br />
Pf. 987.<br />
Hungary<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
Italy AIMDD<br />
Directorate <strong>of</strong><br />
Health<br />
Mrs Anna Bjorg<br />
Austurstrond 5,<br />
Aradottir<br />
IS-170 Seltjarnarnes<br />
Iceland<br />
Irish Medicines<br />
Board. <strong>Medical</strong><br />
<strong>Devices</strong> Department Ms. Andrea Hanson<br />
Earlsfort Centre Ms. Jan Guerin<br />
Earlsfort Terrace<br />
Dublin 2<br />
Ireland<br />
Ministry <strong>of</strong> Health<br />
Dipartimento<br />
dell'innovazione<br />
AIMDD and MDD: -<br />
Ufficio III, Dispositivi<br />
medici -Via della<br />
Civiltà Romana, 7<br />
00144 ROMA<br />
annabara@landlaeknir.is<br />
medicaldevices@<br />
imb.ie<br />
g.ruocco@sanita.it<br />
www.land<br />
laeknir.is<br />
www.imb.ie<br />
3 <strong>of</strong> 6 02.08.2007 10:56
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
Latvia<br />
Liechenstein<br />
(EFTA)<br />
Lithuania<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
Luxembourg MDD<br />
Malta<br />
Netherlands<br />
AIMDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
Dispositivi Medici<br />
Piazzale<br />
dell'Industria 20<br />
0144 Roma<br />
Health Statistics and<br />
<strong>Medical</strong><br />
Technologies State<br />
Agency<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Board for Latvia<br />
12/22 Duntes<br />
street,Riga,<br />
Latvia, LV-1005<br />
Bureau <strong>of</strong> <strong>Medical</strong><br />
<strong>Devices</strong> /IVD<br />
Office <strong>of</strong> Food<br />
Inspection and<br />
Veterinary Affairs<br />
Postplatz 2<br />
LI-9494 Schaan<br />
Vaduz<br />
Liechenstein<br />
dott.Giuseppe<br />
Ruocco<br />
IVCMD: Ufficio IV,<br />
Diagnostici in<br />
vitro-Via della Civiltà<br />
Romana, 7 00144<br />
ROMA<br />
dott.ssa Giovana<br />
Nisticò<br />
Mr. Egils<br />
Lavendelis<br />
The State<br />
Health Care<br />
Accreditation<br />
Agency<br />
under the<br />
Ministry <strong>of</strong><br />
Health <strong>of</strong> the Director:<br />
Republic <strong>of</strong> Pr<strong>of</strong>. Juozas<br />
Lithuania Galdikas<br />
Zalgirio str.<br />
92<br />
LT- 09303<br />
Vilnius<br />
Lithuania<br />
Minist?e de la<br />
Sant?/font><br />
Villa Louvigny ? all?<br />
Marconi<br />
L-2120 Luxembourg<br />
Malta Standards<br />
Authority<br />
Second Floor,<br />
Evans Building,<br />
Merchants Street,<br />
Valletta VLT 03<br />
Malta<br />
AIMDD Legislation<br />
Ministry <strong>of</strong> Health,<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
Welfare and Sport<br />
P.O. Box 20350<br />
2500 EJ The Hague<br />
The Netherlands<br />
Law Enforcement<br />
Healthcare<br />
Inspectorate<br />
g.nistico@sanita.it<br />
agentura@vsmta.lv<br />
Mrs Yvonne Spano Yvonne.Spano-Wellenzohn@alkvw.llv.li<br />
Dr Gérard Scharll<br />
Ing. Anthony<br />
Camilleri<br />
Head <strong>of</strong> Consumer<br />
and Industrial Goods<br />
Directorate<br />
Mrs Sabina<br />
Hoekstra-van den<br />
Bosch, Pharm.D.<br />
juozas.galdikas@vaspvt.sam.lt<br />
Common email:<br />
vaspvt@vaspvt.sam.lt<br />
gerard.scharll@<br />
ms.etat.lu<br />
www.sam.lt/<br />
vaspvt<br />
www.etat.lu/MS/<br />
anthony.camilleri@msa.org.mt www.msa.org.mt<br />
sl.hoekstra@minvws.nl www.minvws.nl<br />
Postal address :<br />
P.O. Box 16119,<br />
2500 BC The Hague<br />
Mr Jos Kraus medtech.higz@igz.nl www.igz.nl<br />
4 <strong>of</strong> 6 02.08.2007 10:56
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
Norway<br />
(EFTA)<br />
Poland<br />
Portugal<br />
Romania<br />
Slovenia<br />
Slovakia<br />
The Netherlands<br />
Visitors address :<br />
Parnassusplein 5,<br />
2511 VX The Hague<br />
The Netherlands<br />
IVDMD Ms L.M. de Vries<br />
AIMDD Directorate for<br />
Health and Social<br />
MDD<br />
Affairs<br />
P.O.Box 7000<br />
ST. Olavs plass<br />
N-0130 Oslo<br />
IVDMD Norway<br />
MDD<br />
AIMD<br />
IVDMD<br />
MDD<br />
AIMD<br />
IVDMD<br />
MDD<br />
AIMD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
Legislation<br />
Designation <strong>of</strong> NBs<br />
Ministry <strong>of</strong> Health<br />
Miodowa 15<br />
00-952 Warsaw<br />
Poland<br />
<strong>Competent</strong> Authority<br />
Office for<br />
Registration <strong>of</strong><br />
Medicinal Products,<br />
<strong>Medical</strong> <strong>Devices</strong><br />
and Biocidal<br />
Products<br />
Zabkowska 41<br />
03 – 736 Warsaw<br />
Poland<br />
Instituto Nacional de<br />
Saude<br />
Dr. Ricardo Jorge<br />
Av. Padre Cruz<br />
1649-016 Lisboa<br />
Portugal<br />
Infarmed<br />
Parque de Saude de<br />
Lisboa<br />
Av. do Brasil, n. 53<br />
1749-004 Lisboa<br />
Portugal<br />
Ministry <strong>of</strong> Health<br />
and Family<br />
1-3, Cristian<br />
Popisteanu Street,<br />
Sector 1<br />
010024, Bucharest<br />
Romania<br />
Ingeborg<br />
Hagerup-Jenssen<br />
Bjørn Kristian Berge<br />
Sissel Dyrnes<br />
meddev-no@shdir.no www.shdir.no<br />
Andrzej Bajor a.bajor@mz.gov.pl www.mz.gov.pl<br />
Joanna Kilkowska<br />
Andrzej Karczewicz<br />
Jose Lavinha,<br />
Pharm.D., Director <strong>of</strong><br />
the Institute<br />
Heitor Costa, M.D.,<br />
Director <strong>of</strong> Human<br />
and Veterinary<br />
Medicines and<br />
Healthcare Products<br />
Judite Neves,<br />
Pharm.D., Head <strong>of</strong><br />
Department <strong>of</strong><br />
<strong>Medical</strong> <strong>Devices</strong><br />
Agency for<br />
Medicinal Products<br />
and <strong>Medical</strong><br />
<strong>Devices</strong> <strong>of</strong> the<br />
Dr Petra Marinko<br />
Republic <strong>of</strong> Slovenia<br />
Ptujska ulica 21<br />
SI-1000 Ljubljana<br />
Slovenia<br />
State Institute for<br />
Drug Control<br />
joanna.kilkowska@urpl.gov.pl<br />
andrzej.karczewicz@urpl.gov.pl<br />
www.urpl.gov.pl<br />
info@insa.min-saude.pt www.insarj.pt<br />
daps@infarmed.pt www.infarmed.pt<br />
Ana Tanase atanase@ms.ro www.ms.ro<br />
meddev@jazmp.si<br />
petra.marinko@jazmp.si<br />
www.jazmp.si<br />
5 <strong>of</strong> 6 02.08.2007 10:56
<strong>Enterprise</strong> - <strong>Medical</strong> <strong>Devices</strong> - <strong>List</strong> <strong>of</strong> <strong>Competent</strong> <strong>Authorities</strong> http://ec.europa.eu/enterprise/medical_devices/ca/list_ca.htm<br />
Spain<br />
Sweden<br />
Switzerland<br />
(EFTA)<br />
Turkey<br />
(Candidate)<br />
United<br />
Kingdom<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
SectionKvetna 11,<br />
825 08 Bratislava 26<br />
Slovak Republic<br />
Mr. Marek Slávik<br />
Ministerio Sanidad y<br />
Consumo<br />
Agencia Espanola<br />
de Medicamentos y<br />
Productos<br />
Sanitarios<br />
Parque Empresarial<br />
Las Mercedes.<br />
Edificio 8. C/<br />
Campezo 1<br />
28022 Madrid<br />
Spain<br />
<strong>Medical</strong> Products<br />
Agency<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Box 26<br />
SE-751 03 Uppsala<br />
Sweden<br />
deak@sukl.sk<br />
slavik@sukl.sk<br />
Mrs. C. Abad sgps@agemed.es<br />
Dr. Lennart Philipson<br />
Mr. Lars Johansson<br />
AIMDD<br />
MDD<br />
Swissmedic<br />
<strong>Medical</strong> <strong>Devices</strong><br />
Division<br />
Erlachstr. 8<br />
Mrs. Margit Widmann<br />
IVDMD<br />
CH 3000 Bern 9<br />
Switzerland<br />
Mr. Andreas Schlegel<br />
AIMDD<br />
MDD<br />
IVDMD<br />
AIMDD<br />
MDD<br />
IVDMD<br />
Last update: 18/08/2006<br />
Medicines and<br />
Healthcare products<br />
Regulatory Agency<br />
(MHRA)<br />
8th Floor<br />
Market Towers<br />
1 Nine Elms Road<br />
London SW8 6NQ<br />
United Kingdom<br />
meddev.central@mpa.se<br />
margit.widmann@swissmedic.ch<br />
medical.devices@swissmedic.ch<br />
andreas.schlegel@swissmedic.ch<br />
medical.devices@swissmedic.ch<br />
Mr. Steve Owen steve.owen@mhra.gsi.gov.uk<br />
www.msc.es<br />
www.agemed.es<br />
http://www.lakemedelsverket.se/<br />
www.swissmedic<br />
.ch/md.asp<br />
www.mhra.<br />
gov.uk<br />
6 <strong>of</strong> 6 02.08.2007 10:56