- Page 2: The Antioxidant Vitamins C and E Ed
- Page 6: Acknowledgment The Oxygen Club of C
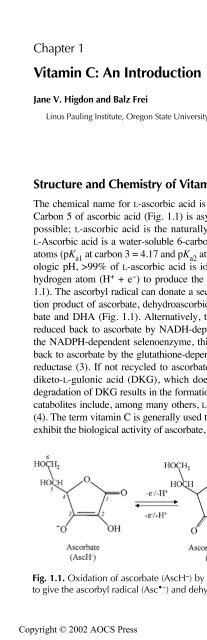
- Page 10: Contents Chapter 1 Vitamin C: An In
- Page 16: Biological Activities of Vitamin C
- Page 20: iron absorption (21). Further resea
- Page 24: established, the optimal intake of
- Page 28: Recognition of the Role of Vitamin
- Page 32: ascorbate concentrations observed i
- Page 36: 4. Rumsey, S.C., and Levine, M. (19
- Page 40: 41. Park, J.B., and Levine, M. (200
- Page 44: 76. Eye Disease Case Control Study
- Page 48: C dose; and outpatient or uncertain
- Page 52: Fig. 2.1. Vitamin C plasma concentr
- Page 56: Fig. 2.3. Steady-state plasma vitam
- Page 60: Examples of data obtained for bioav
- Page 64:
Fig. 2.6. Urinary vitamin C excreti
- Page 68:
ment of life-threatening meningitis
- Page 72:
(1971) Metabolism of 14 C- and 3 H-
- Page 76:
Chapter 3 Biochemical and Physiolog
- Page 80:
in vitro experiments have shown tha
- Page 84:
Many studies have shown that iron c
- Page 88:
Lipid peroxidation. The study of li
- Page 92:
oxidation reactions in proteins are
- Page 96:
to the presence of metal binding pr
- Page 100:
plementation period (Table 3.2). Ho
- Page 104:
Aqueous Dispersions of Lipids: Unre
- Page 108:
55. Anderson, D., Yu, T.W., Phillip
- Page 112:
Chapter 4 Vitamin C and Cancer Seon
- Page 116:
mutase, catalase, and reduced gluta
- Page 120:
4-hydroperoxy-2-nonenal and a conco
- Page 124:
Fig. 4.4. Potential formation of DN
- Page 128:
Fig. 4.7. Liquid chromatography/mas
- Page 132:
eluted with methanol/water. The elu
- Page 136:
adducts then dehydrate to a single
- Page 140:
Pathways of Formation of 4-Hydroxyn
- Page 144:
Chapter 5 Ascorbic Acid and Endothe
- Page 148:
NO synthesis (67-70). Tetrahydrobio
- Page 152:
Statistical analysis. All data are
- Page 156:
Fig. 5.2. Time-dependence of the up
- Page 160:
Fig. 5.4. Influence of sepiapterin
- Page 164:
Fig. 5.6. Effect of ascorbic acid o
- Page 168:
Fig. 5.8. Effect of ascorbic acid o
- Page 172:
Fig. 5.10. Effect of ascorbic acid
- Page 176:
5. Cayatte, A.J., Placino, J.J., Ho
- Page 180:
Dysfunction of Epicardial Coronary
- Page 184:
Impact on Nitric Oxide-Mediated For
- Page 188:
99. Gal, E.M., Nelson, J.M., and Sh
- Page 192:
Serum Ascorbic Acid and Cardiovascu
- Page 196:
Serum Ascorbic Acid, Bone Mineral D
- Page 200:
Serum Ascorbic Acid and Lead Poison
- Page 204:
Fig. 6.2. The relation between seru
- Page 208:
TABLE 6.2 Relation of Serum Ascorbi
- Page 212:
9. Harats, D., Ben-Naim, M., Dabach
- Page 216:
49. Committee on Diet and Health, F
- Page 220:
86. Bayeta, E., and Lau, B.H.S. (20
- Page 224:
min C plays in the etiology of CVD
- Page 228:
TABLE 7.1 Prospective Cohort Studie
- Page 232:
TABLE 7.2 Prospective Cohort Studie
- Page 236:
ment for CHD risk factors and intak
- Page 240:
References 1. Jacob, R.A. (1994) Vi
- Page 244:
Chapter 8 Potential Adverse Effects
- Page 248:
educed risk of all-cause mortality
- Page 252:
supplementation, respectively) (23)
- Page 256:
ous form, which is active in the Fe
- Page 260:
Other Adverse Effects Several other
- Page 264:
termed, a redox imbalance) might oc
- Page 268:
and Willett, W.C. (1999) Relation o
- Page 272:
Peripheral Lymphocytes: A Case Obse
- Page 276:
85. Brown, K.M., Morrice, P.C., and
- Page 280:
Fig. 9.1. The molecular structure o
- Page 284:
Although universal dietary suppleme
- Page 288:
ac-α-tocopherol all exhibit vitami
- Page 292:
equires NADPH- and NADH-dependent r
- Page 296:
TABLE 9.1 Ongoing Clinical Trials I
- Page 300:
TABLE 9.1 (continued) Ongoing Clini
- Page 304:
15. Jiang, Q., Christen, S., Shigen
- Page 308:
52. Rhoden, E.L., Pereira-Lima, L.,
- Page 312:
Glomerular Dysfunction in Diabetic
- Page 316:
Chapter 10 Bioavailability and Biop
- Page 320:
amin E activity of α-tocopherol is
- Page 324:
Fig. 10.2. Ratios of RRR:all-rac-α
- Page 328:
Fig. 10.3. Ratios of d 3 -RRR:d 6 -
- Page 332:
20. Burton, G.W., Ingold, K.U., Che
- Page 336:
Lack of Bioequivalence of RRR- and
- Page 340:
Ttpa +/+ and Ttpa +/− mice were d
- Page 344:
Vitamin E Kinetics During Pregnancy
- Page 348:
transported, and excreted by nonspe
- Page 352:
Following α-, γ-, or δ-Tocotrien
- Page 356:
Sorting In the liver, α-TTP select
- Page 360:
SCHEME 12.2. Mechanism of γ-tocotr
- Page 364:
Fig. 12.1. Rifampicin stimulates th
- Page 368:
3. O’Byrne, D., Grundy, S., Packe
- Page 372:
Chapter 13 γ-Tocopherol Metabolism
- Page 376:
γγ-Tocopherol Metabolism A number
- Page 380:
HPLC Analysis of Plasma αα- and
- Page 384:
The final urinary CEHC and QL conce
- Page 388:
Fig. 13.3. Mean urinary metabolite
- Page 392:
Fig. 13.5. The d 0 and d 2 plasma
- Page 396:
2. Behrens, W.A, and Madere, R. (19
- Page 400:
33. Kelly, F.J., Rodgers, W., Handl
- Page 404:
Controlled Intervention Trials The
- Page 408:
of α-tocopherol appears to represe
- Page 412:
Fig. 14.2. Effect of α-tocopherol
- Page 416:
References 1. Ben Hamida, C., Doerf
- Page 420:
29. Tamaru, Y., Hirano, M., Kusaka,
- Page 424:
60. Febbraio, M., Podrez, E., Smith
- Page 428:
of RRR-α-Tocopherol and all-Racemi
- Page 432:
obtain a more comprehensive underst
- Page 436:
TABLE 15.2 Selection of Se- and Vit
- Page 440:
endothelial cell metabolism describ
- Page 444:
Chapter 16 Vitamin E and Enhancemen
- Page 448:
y serving as a substrate for the en
- Page 452:
TABLE 16.2 Effects of O 2 •− ,
- Page 456:
high-affinity IL-2 receptors by its
- Page 460:
(1) reported that naive PCC-specifi
- Page 464:
11. Gately, M.K., Renzetti, L.M., M
- Page 468:
Chapter 17 Is There a Role for Vita
- Page 472:
min E has been reported to signific
- Page 476:
TABLE 17.1 Vitamin E and CVD: Prima
- Page 480:
TABLE 17.3 Cardiovascular Disease (
- Page 484:
significantly affecting aortic conc
- Page 488:
synthase genotype in the CHAOS stud
- Page 492:
23. Hazell, L.J., and Stocker, R. (
- Page 496:
Linoleate Hydroperoxides in Oxidize
- Page 500:
assigned to one of the three regime
- Page 504:
an English diet; the CHAOS trial us
- Page 508:
of high-dose AT supplementation on
- Page 512:
The strengths of this study were th
- Page 516:
prevention; the benefit that other
- Page 520:
19. Mitchinson, M.J., Stephens, N.G
- Page 524:
normal or increased vitamin E level
- Page 528:
Age-Related Macular Degeneration (A
- Page 532:
proven beneficial for patients with
- Page 536:
in plasma of patients given intrave
- Page 540:
tions in the brain can be increased
- Page 544:
Skin Aging (Photoaging) The aging o
- Page 548:
of photodamage such as erythema in
- Page 552:
esponse trial among healthy older a
- Page 556:
The effects of vitamin E and relate
- Page 560:
and Tocopherols in Preeclamptic and
- Page 564:
42. Eye Disease Case Control Study
- Page 568:
71. Bonfigli, A.R., Pieri, C., Manf
- Page 572:
103. Kinalski, M., Sledziewski, A.,
- Page 576:
133. Aparicio, J.M., Belanger-Quint
- Page 580:
162. Masaki, K.H., Losonczy, K.G.,
- Page 584:
196. Dreher, F., Gabard, B., Schwin
- Page 588:
230. Child, R.B., Wilkinson, D.M.,