Safe-T-Centesis® and Safe-T-Centesis PLUS - CareFusion
Safe-T-Centesis® and Safe-T-Centesis PLUS - CareFusion
Safe-T-Centesis® and Safe-T-Centesis PLUS - CareFusion
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
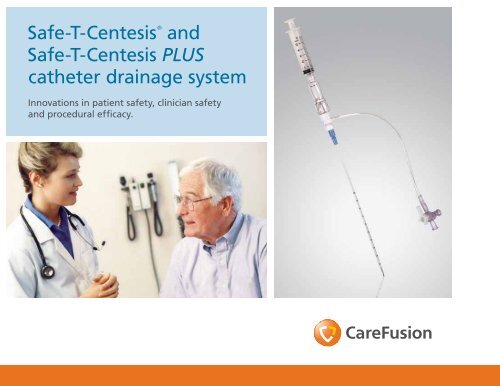
<strong>Safe</strong>-T-<strong>Centesis</strong> ®<br />
<strong>and</strong><br />
<strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong><br />
catheter drainage system<br />
Innovations in patient safety, clinician safety<br />
<strong>and</strong> procedural efficacy.
<strong>Safe</strong>-T-<strong>Centesis</strong> catheter drainage system<br />
This innovative drainage system is designed to reduce the risks associated with normal percutaneous needle<br />
placements <strong>and</strong> enhance patient comfort <strong>and</strong> procedural flexibility.<br />
Visual color indicator<br />
Transitions to red when sharp distal tip<br />
is exposed <strong>and</strong> returns to “<strong>Safe</strong>-White”<br />
when spring-loaded obturator is<br />
automatically extended, presenting<br />
an atraumatic front to injury.<br />
Self-sealing valve<br />
Maintains closed system or allows for reinsertion of<br />
introducer needle or guide wire for procedures not<br />
requiring maintenance of a closed system.<br />
Silicone coating<br />
Allows for smooth percutaneous insertion while<br />
minimizing potential for “accordion” effect <strong>and</strong><br />
reducing tissue trauma.<br />
Pigtail catheter<br />
Self-directing <strong>and</strong> occlusion-resistant.<br />
Centimeter depth markings<br />
Reliable reference for safe catheter placement<br />
with 16 cm of usable catheter length.<br />
Catheter modularity<br />
For procedures not requiring maintenance of a closed<br />
system, the luer lock connection allows convenient<br />
removal of pigtail catheter from valve assembly.<br />
Minimally invasive 6 Fr or 8 Fr size<br />
Remote stopcock<br />
Provides procedural convenience <strong>and</strong><br />
safety by facilitating connection to<br />
drainage/extension sets while minimizing<br />
inadvertent device or catheter movement.<br />
Veress technology duallumen<br />
blunt obturator<br />
Provides patient protection from<br />
perforations <strong>and</strong> user protection<br />
from needlestick injuries.
Innovations for patient safety <strong>and</strong> procedural efficacy<br />
Our combination of unique features provides patient protection from<br />
perforations <strong>and</strong> clinician protection from needlestick injuries.<br />
Veress technology with<br />
visual color indicator<br />
Diagram A<br />
1. The color change indicator confirms<br />
“<strong>Safe</strong>-White” when the blunted obturator<br />
is extended over the sharp distal tip.<br />
2. Prior to initial anatomical contact or<br />
when the distal tip of the device clears an<br />
anatomical obstruction or enters a free<br />
cavity, the spring-loaded obturator will<br />
automatically advance forward, providing<br />
protection against patient injury.<br />
Diagram B<br />
3. The color change indicator informs<br />
the user of anatomical contact by<br />
transitioning from “<strong>Safe</strong>-White” to<br />
“Caution-Red.”<br />
4. The blunted obturator automatically<br />
retracts upon contact with an anatomical<br />
structure, exposing the sharp distal tip<br />
<strong>and</strong> allowing needle advancement.<br />
Diagram A Diagram B<br />
1 3<br />
2 4<br />
Pigtail catheter<br />
• Pigtail shape for secure placement <strong>and</strong><br />
anatomically assisted self-direction<br />
• Occlusion-resistant, with four ports<br />
strategically placed on inside apex<br />
of pigtail<br />
• Silicone coated for smooth insertion <strong>and</strong><br />
minimal potential for “accordion” effect<br />
Self-sealing valve<br />
• Closed system for minimized risk of<br />
pneumothorax or leakage<br />
• The resealing membrane within the<br />
valve system allows the safety introducer<br />
needle assembly to be reinserted<br />
(for procedures not requiring maintenance<br />
of a closed system)<br />
• Compatible with 0.035 <strong>and</strong> 0.038 guide<br />
wires to clear occlusions or exp<strong>and</strong><br />
your procedural capabilities beyond the<br />
percutaneous realm<br />
(for procedures not requiring maintenance<br />
of a closed system)<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> catheter drainage system
<strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> catheter drainage system<br />
Our pigtail catheter drainage system is also available in a <strong>Safe</strong>-T <br />
<strong>PLUS</strong> tray, designed to help you comply<br />
with today’s safety requirements <strong>and</strong> recommendations.<br />
Healthcare organization Recommendation<br />
The Joint Commission<br />
Centers for Disease Control &<br />
Prevention (CDC)<br />
National Institute for Occupational<br />
<strong>Safe</strong>ty <strong>and</strong> Health (NIOSH)<br />
Occupational <strong>Safe</strong>ty <strong>and</strong> Health<br />
Administration (OSHA)<br />
International Sharps Injury<br />
Prevention Society (ISIPS)<br />
Centers for Disease Control &<br />
Prevention (CDC)<br />
Institute for Healthcare<br />
Improvement (IHI)<br />
Centers for Disease Control &<br />
Prevention (CDC)<br />
Preventable medical error reduction<br />
2006 National Patient <strong>Safe</strong>ty Goals<br />
Label all medications, medication containers or other solutions on <strong>and</strong> off the sterile field. 1<br />
2003 National Patient <strong>Safe</strong>ty Goals<br />
Universal protocol to prevent wrong site, procedure or surgery, including “Time Out” immediately before starting<br />
any surgical or invasive procedure, to conduct a final verification process to confirm the correct patient, procedure<br />
<strong>and</strong> site using active not passive communication techniques. 2<br />
Sharps safety<br />
The CDC estimates that each year 385,000 needlesticks <strong>and</strong> other sharps-related injuries are sustained by hospitalbased<br />
healthcare personnel; an average of 1,000 sharps injuries per day. 3<br />
Sharps injury prevention devices should include either sharps disposal containers <strong>and</strong> needles or other devices with<br />
an integrated engineered sharps injury prevention feature. 4<br />
2001 revision of Bloodborne Pathogens & Needlestick Prevention st<strong>and</strong>ards<br />
<strong>Safe</strong>r needle devices that reflect technology changes to eliminate or reduce exposure to bloodborne pathogens. 5<br />
Promote services <strong>and</strong> products that encompass the entire universe of safety products from modified sharps to<br />
alternative products that actually eliminate the sharp. 6<br />
Infection prevention<br />
The CDC recognized that “In the United States, povidone iodine has been the most widely used antiseptic for<br />
cleansing arterial catheter <strong>and</strong> CVC-insertion sites. However, in one study, preparation of central venous <strong>and</strong> arterial<br />
sites with a 2% aqueous chlorhexdine gluconate lowered BSI rates compared with site preparation with 10%<br />
povidone-iodine or 70% alcohol.” 7<br />
Protecting 5 Million Lives From Harm<br />
One goal of this campaign is to help prevent CRBSIs through implementing a set of five interventions known as the<br />
“Central Line Bundle.” One of these is chlorhexidine skin antisepsis. 8<br />
Another intervention recommended by IHI is maximal barrier precautions. 8<br />
The CDC now recommends maximum sterile barrier precautions, including use of cap, mask, sterile gown, sterile<br />
gloves <strong>and</strong> large sterile drape during the insertion of CVCs. These substantially reduce the incidence of CRBSI. 9<br />
<strong>Safe</strong>-T-<strong>Centesis</strong><br />
<strong>PLUS</strong> tray solution<br />
Medication<br />
labels <strong>and</strong> fine<br />
tip marker<br />
Time out label<br />
BD <strong>Safe</strong>tyGlide <br />
needles<br />
BD Bard Parker <br />
protected<br />
disposable scalpel<br />
Filter Straw ®<br />
ChloraPrep ®<br />
3 mL applicator<br />
(Hi-Lite Orange ® tint)<br />
Facial protection
<strong>Safe</strong>-T <strong>PLUS</strong> components<br />
<strong>Safe</strong>-T <strong>PLUS</strong> trays assist with compliance for preventable medical error reduction,<br />
sharps safety <strong>and</strong> infection prevention, <strong>and</strong> feature these safety components:<br />
1. Medication labels <strong>and</strong> fine tip marker can be used on syringes, medication cups or basins to identify<br />
medications being used on the sterile field. The waterproof label helps eliminate concerns if there is a spill<br />
on the sterile field.<br />
2. Time out label is designed to prompt the user to verify the Correct Patient, Correct Site <strong>and</strong> Correct Procedure.<br />
Since this label is also a sticker, it allows the user to place a completed label in the patient chart for good record<br />
keeping practices.<br />
3. BD <strong>Safe</strong>tyGlide needle with BD Activation-Assist <br />
technology allows for fast <strong>and</strong> easy, single-h<strong>and</strong>ed<br />
needle shielding. It can help prevent needlestick injuries by allowing easy <strong>and</strong> secure shielding of the needle<br />
tip immediately after injection.<br />
4. BD Bard-Parker protected disposable scalpel with quality stainless-steel blades <strong>and</strong> safety engineering<br />
delivers performance without compromise to technique. They are designed with the h<strong>and</strong>ling <strong>and</strong> feel that<br />
clinicians prefer, with simple one-h<strong>and</strong>ed activation <strong>and</strong> protection before <strong>and</strong> after use. In addition, they help<br />
your facility to comply with OSHA <strong>and</strong> federal safety legislation.<br />
5. Filter Straw particulate matter filter allows the user to access medication contained in an ampoule while<br />
filtering out glass particles that may have entered during opening.<br />
6. Needle foam stop is designed for added sharps safety (in addition to the Poink-Lok device).<br />
7. ChloraPrep solution is a rapid-acting, broad spectrum <strong>and</strong> persistent patient preoperative skin preparation<br />
in a single-use sterile applicator. ChloraPrep solution combines 2% chlorhexidine gluconate (CHG) <strong>and</strong> 70%<br />
isopropyl alcohol (IPA) in a patented applicator for a proven system that supports infection control guidelines to<br />
help reduce the incidence of surgical site infections. Hi-Lite Orange tint provides visual marker of prepped site.<br />
8. Facial protection with a comfortable, easy to don, one-size fits all mask. Allows the user to exercise barrier<br />
precautions during a procedure.<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7<br />
8<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> catheter drainage system
Ordering information<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> catheter drainage system<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> catheter<br />
drainage tray<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> trays contain the necessary<br />
components you need for a procedure,<br />
including safety components, all in one place.<br />
Cat no. Description<br />
PIG1260T <strong>Safe</strong>-T-<strong>Centesis</strong> 6 Fr catheter<br />
drainage tray<br />
PIG1280T <strong>Safe</strong>-T-<strong>Centesis</strong> 8 Fr catheter<br />
drainage tray<br />
Tray components<br />
• Needle-Pro ® needle, 25G x ˝ (1.6 cm) with<br />
needle protection device<br />
• Needle-Pro needle, 22G x 1½˝ (3.8 cm) with<br />
needle protection device<br />
• Two Point-Lok ® needle retention safety devices<br />
• Futura retractable safety scalpel<br />
• Filter needle, 19G x 1½˝ (3.8 cm)<br />
• Luer lock syringe, 5 mL<br />
• Luer lock syringe, 10 mL<br />
• Luer lock syringe, 60 mL<br />
• Universal drainage set<br />
• Fluid collection bag<br />
• Vacuum extension set with needle<br />
• Syringe protector<br />
• Fluid collection protector<br />
• Three povidone-iodine swab sticks,<br />
1% available iodine<br />
• Two pre-labeled 10 mL specimen<br />
vials with caps<br />
• Six gauze pads<br />
• Wall suction adapter<br />
• Two ampoules of 1% lidocaine hydrochloride,<br />
USP, 5 mL<br />
• Fenestrated drape<br />
• Towel<br />
• Hospital wrap<br />
• B<strong>and</strong>age<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> catheter<br />
drainage kit<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> kits provide a simplified<br />
format while maintaining important safety<br />
components to meet a variety of needs.<br />
Cat no. Description<br />
PIG1260K <strong>Safe</strong>-T-<strong>Centesis</strong> 6 Fr catheter<br />
drainage kit<br />
PIG1280K <strong>Safe</strong>-T-<strong>Centesis</strong> 8 Fr catheter<br />
drainage kit<br />
Kit components<br />
• Vacuum extension set with needle<br />
• Futura retractable safety scalpel<br />
• Point-Lok needle retention safety device
Ordering information<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> catheter drainage system<br />
<strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> catheter drainage tray<br />
The <strong>Safe</strong>-T <strong>PLUS</strong> tray featuring the <strong>Safe</strong>-T-<strong>Centesis</strong> device is<br />
designed to assist with compliance with today’s safety requirements<br />
<strong>and</strong> recommendations.<br />
Cat no. Description<br />
PIG1260TSP <strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> 6 Fr catheter drainage tray<br />
PIG1280TSP <strong>Safe</strong>-T-<strong>Centesis</strong> <strong>PLUS</strong> 8 Fr catheter drainage tray<br />
Tray components<br />
• Fluid collection bag<br />
• Universal drainage set<br />
• Drainage tube with 15G x 1½”<br />
vacuum needle<br />
• Wall suction adapter<br />
• Three prelabeled specimen tubes<br />
with caps, 10 mL<br />
• Lidocaine HCL USP, 1%, 5 mL<br />
• Luer lock syringe, 10 mL<br />
• Luer lock syringe, 60 mL<br />
• Gauze pads, 3 x 3” 8 ply<br />
• B<strong>and</strong>age<br />
• Towel, 19” x 16”<br />
• Fenestrated drape with tape<br />
<strong>Safe</strong>-T <strong>PLUS</strong> components<br />
• BD <strong>Safe</strong>tyGlide 22G x 1½”<br />
• BD <strong>Safe</strong>tyGlide 25G x 1”<br />
• BD protected disposable<br />
scalpel, #11<br />
• ChloraPrep 3 mL applicator with<br />
Hi-Lite Orange tint<br />
• Point-Lok needle retention safety<br />
device<br />
• Filter Straw filter<br />
• Medication label sheet<br />
• Fine tip marker<br />
• Time-out label<br />
• Needle foam stop<br />
• Ear loop mask<br />
Ordering information
1 Joint Commission on the Accreditation of Healthcare Organizations. 2006 National Patient <strong>Safe</strong>ty Goals. Implementation Expectations for the 2007 NPSG’s PDF.<br />
Available at: http://www.jcipatientsafety.org/22847/. Accessed April 25, 2008.<br />
2 Joint Commission on the Accreditation of Healthcare Organizations. 2003 National Patient <strong>Safe</strong>ty Goals. Implementation Expectations for Time Out PDF.<br />
Available at: http://www.jcipatientsafety.org/22848/. Accessed April 25, 2008.<br />
3 Centers for Disease Control & Prevention. Workbook for Designing, Implementing, <strong>and</strong> Evaluating a Sharps Injury Prevention Program.<br />
Available at: http://www.cdc.gov/sharpssafety/wk_info.html. Accessed April 25, 2008.<br />
4 National Institute for Occupational <strong>Safe</strong>ty <strong>and</strong> Health. Workbook for Designing, Implementing, <strong>and</strong> Evaluating a Sharps Injury Prevention Program.<br />
Available at: http://www.cdc.gov/sharpssafety/wk_overview.html#overViewIntro. Accessed April 25, 2008.<br />
5 Occupational <strong>Safe</strong>ty <strong>and</strong> Health Administration. 2001 revision of Bloodborne Pathogens & Needlestick Prevention St<strong>and</strong>ards. Bloodborne Pathogens <strong>and</strong><br />
Needlestick Prevention OSHA St<strong>and</strong>ards. Available at: http://www.osha.gov/SLTC/bloodbornepathogens/st<strong>and</strong>ards.html. Accessed April 25, 2008.<br />
6 International Sharps Injury Prevention Society. Guidelines for the Prevention of Intravascular Catheter-Related Infections.<br />
Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed April 25, 2008.<br />
7 Centers for Disease Control <strong>and</strong> Prevention. In the August 9th, 2002 issue of Morbidity <strong>and</strong> Mortality Weekly Report.<br />
Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed April 25, 2008.<br />
8 Institute for Healthcare Improvement. Protecting 5 Million Lives from Harm, Prevent Central Line-Associated Bloodstream Infections.<br />
One page summary download Available at: http://www.ihi.org/IHI/Programs/Campaign/CentralLineInfection.htm. Accessed April 25, 2008.<br />
9 CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections.<br />
Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5110a1.htm. Accessed April 25, 2008.<br />
For more information or to place an order, please contact your Interventional Specialties<br />
Sales Representative, call 800.653.6827 or visit carefusion.com/interventional.<br />
<strong>CareFusion</strong><br />
Waukegan, IL<br />
carefusion.com<br />
© 2010 <strong>CareFusion</strong> Corporation or one of its subsidiaries. All rights reserved. ChloraPrep, Hi-Lite Orange, <strong>Safe</strong>-T <strong>and</strong> <strong>Safe</strong>-T-<strong>Centesis</strong> are trademarks or registered trademarks of<br />
<strong>CareFusion</strong> Corporation or one of its subsidiaries. All other trademarks are property of their respective owners. IS1888 (2000/1110)