Transmutation of metal at low energy in a confined ... - LENR-CANR
Transmutation of metal at low energy in a confined ... - LENR-CANR
Transmutation of metal at low energy in a confined ... - LENR-CANR
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
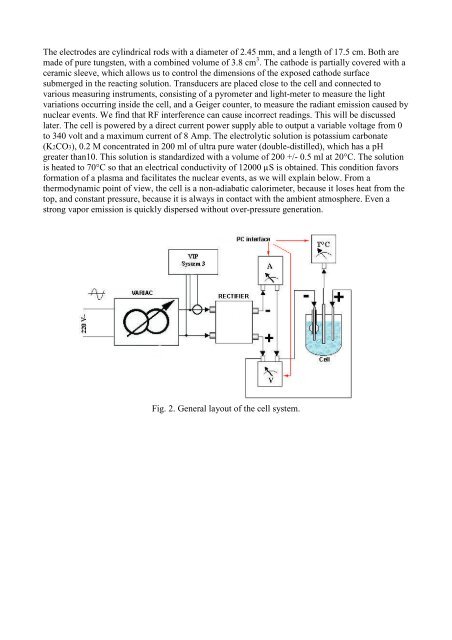
The electrodes are cyl<strong>in</strong>drical rods with a diameter <strong>of</strong> 2.45 mm, and a length <strong>of</strong> 17.5 cm. Both are<br />
made <strong>of</strong> pure tungsten, with a comb<strong>in</strong>ed volume <strong>of</strong> 3.8 cm 3 . The c<strong>at</strong>hode is partially covered with a<br />
ceramic sleeve, which al<strong>low</strong>s us to control the dimensions <strong>of</strong> the exposed c<strong>at</strong>hode surface<br />
submerged <strong>in</strong> the react<strong>in</strong>g solution. Transducers are placed close to the cell and connected to<br />
various measur<strong>in</strong>g <strong>in</strong>struments, consist<strong>in</strong>g <strong>of</strong> a pyrometer and light-meter to measure the light<br />
vari<strong>at</strong>ions occurr<strong>in</strong>g <strong>in</strong>side the cell, and a Geiger counter, to measure the radiant emission caused by<br />
nuclear events. We f<strong>in</strong>d th<strong>at</strong> RF <strong>in</strong>terference can cause <strong>in</strong>correct read<strong>in</strong>gs. This will be discussed<br />
l<strong>at</strong>er. The cell is powered by a direct current power supply able to output a variable voltage from 0<br />
to 340 volt and a maximum current <strong>of</strong> 8 Amp. The electrolytic solution is potassium carbon<strong>at</strong>e<br />
(K2CO3), 0.2 M concentr<strong>at</strong>ed <strong>in</strong> 200 ml <strong>of</strong> ultra pure w<strong>at</strong>er (double-distilled), which has a pH<br />
gre<strong>at</strong>er than10. This solution is standardized with a volume <strong>of</strong> 200 +/- 0.5 ml <strong>at</strong> 20°C. The solution<br />
is he<strong>at</strong>ed to 70°C so th<strong>at</strong> an electrical conductivity <strong>of</strong> 12000 µS is obta<strong>in</strong>ed. This condition favors<br />
form<strong>at</strong>ion <strong>of</strong> a plasma and facilit<strong>at</strong>es the nuclear events, as we will expla<strong>in</strong> be<strong>low</strong>. From a<br />
thermodynamic po<strong>in</strong>t <strong>of</strong> view, the cell is a non-adiab<strong>at</strong>ic calorimeter, because it loses he<strong>at</strong> from the<br />
top, and constant pressure, because it is always <strong>in</strong> contact with the ambient <strong>at</strong>mosphere. Even a<br />
strong vapor emission is quickly dispersed without over-pressure gener<strong>at</strong>ion.<br />
Fig. 2. General layout <strong>of</strong> the cell system.