Cryopreservation of Quercus suber somatic embryos - Tree Physiology
Cryopreservation of Quercus suber somatic embryos - Tree Physiology
Cryopreservation of Quercus suber somatic embryos - Tree Physiology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1846 FERNANDES ET AL.<br />
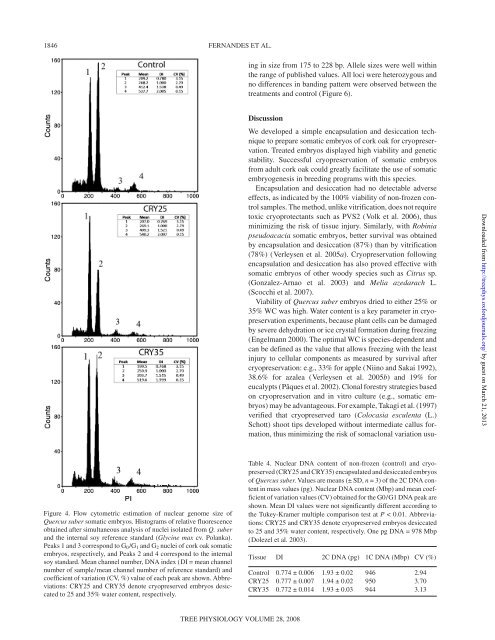
Figure 4. Flow cytometric estimation <strong>of</strong> nuclear genome size <strong>of</strong><br />
<strong>Quercus</strong> <strong>suber</strong> <strong>somatic</strong> <strong>embryos</strong>. Histograms <strong>of</strong> relative fluorescence<br />
obtained after simultaneous analysis <strong>of</strong> nuclei isolated from Q. <strong>suber</strong><br />
and the internal soy reference standard (Glycine max cv. Polanka).<br />
Peaks 1 and 3 correspond to G0/G1 and G2 nuclei <strong>of</strong> cork oak <strong>somatic</strong><br />
<strong>embryos</strong>, respectively, and Peaks 2 and 4 correspond to the internal<br />
soy standard. Mean channel number, DNA index (DI = mean channel<br />
number <strong>of</strong> sample/mean channel number <strong>of</strong> reference standard) and<br />
coefficient <strong>of</strong> variation (CV, %) value <strong>of</strong> each peak are shown. Abbreviations:<br />
CRY25 and CRY35 denote cryopreserved <strong>embryos</strong> desiccated<br />
to 25 and 35% water content, respectively.<br />
TREE PHYSIOLOGY VOLUME 28, 2008<br />
ing in size from 175 to 228 bp. Allele sizes were well within<br />
the range <strong>of</strong> published values. All loci were heterozygous and<br />
no differences in banding pattern were observed between the<br />
treatments and control (Figure 6).<br />
Discussion<br />
We developed a simple encapsulation and desiccation technique<br />
to prepare <strong>somatic</strong> <strong>embryos</strong> <strong>of</strong> cork oak for cryopreservation.<br />
Treated <strong>embryos</strong> displayed high viability and genetic<br />
stability. Successful cryopreservation <strong>of</strong> <strong>somatic</strong> <strong>embryos</strong><br />
from adult cork oak could greatly facilitate the use <strong>of</strong> <strong>somatic</strong><br />
embryogenesis in breeding programs with this species.<br />
Encapsulation and desiccation had no detectable adverse<br />
effects, as indicated by the 100% viability <strong>of</strong> non-frozen control<br />
samples. The method, unlike vitrification, does not require<br />
toxic cryoprotectants such as PVS2 (Volk et al. 2006), thus<br />
minimizing the risk <strong>of</strong> tissue injury. Similarly, with Robinia<br />
pseudoacacia <strong>somatic</strong> <strong>embryos</strong>, better survival was obtained<br />
by encapsulation and desiccation (87%) than by vitrification<br />
(78%) (Verleysen et al. 2005a). <strong>Cryopreservation</strong> following<br />
encapsulation and desiccation has also proved effective with<br />
<strong>somatic</strong> <strong>embryos</strong> <strong>of</strong> other woody species such as Citrus sp.<br />
(Gonzalez-Arnao et al. 2003) and Melia azedarach L.<br />
(Scocchi et al. 2007).<br />
Viability <strong>of</strong> <strong>Quercus</strong> <strong>suber</strong> <strong>embryos</strong> dried to either 25% or<br />
35% WC was high. Water content is a key parameter in cryopreservation<br />
experiments, because plant cells can be damaged<br />
by severe dehydration or ice crystal formation during freezing<br />
(Engelmann 2000). The optimal WC is species-dependent and<br />
can be defined as the value that allows freezing with the least<br />
injury to cellular components as measured by survival after<br />
cryopreservation: e.g., 33% for apple (Niino and Sakai 1992),<br />
38.6% for azalea (Verleysen et al. 2005b) and 19% for<br />
eucalypts (Pâques et al. 2002). Clonal forestry strategies based<br />
on cryopreservation and in vitro culture (e.g., <strong>somatic</strong> <strong>embryos</strong>)<br />
may be advantageous. For example, Takagi et al. (1997)<br />
verified that cryopreserved taro (Colocasia esculenta (L.)<br />
Schott) shoot tips developed without intermediate callus formation,<br />
thus minimizing the risk <strong>of</strong> somaclonal variation usu-<br />
Table 4. Nuclear DNA content <strong>of</strong> non-frozen (control) and cryopreserved<br />
(CRY25 and CRY35) encapsulated and desiccated <strong>embryos</strong><br />
<strong>of</strong> <strong>Quercus</strong> <strong>suber</strong>. Values are means (± SD, n = 3) <strong>of</strong> the 2C DNA content<br />
in mass values (pg). Nuclear DNA content (Mbp) and mean coefficient<br />
<strong>of</strong> variation values (CV) obtained for the G0/G1 DNA peak are<br />
shown. Mean DI values were not significantly different according to<br />
the Tukey-Kramer multiple comparison test at P < 0.01. Abbreviations:<br />
CRY25 and CRY35 denote cryopreserved <strong>embryos</strong> desiccated<br />
to 25 and 35% water content, respectively. One pg DNA = 978 Mbp<br />
(Dolezel et al. 2003).<br />
Tissue DI 2C DNA (pg) 1C DNA (Mbp) CV (%)<br />
Control 0.774 ± 0.006 1.93 ± 0.02 946 2.94<br />
CRY25 0.777 ± 0.007 1.94 ± 0.02 950 3.70<br />
CRY35 0.772 ± 0.014 1.93 ± 0.03 944 3.13<br />
Downloaded from<br />
http://treephys.oxfordjournals.org/ by guest on March 21, 2013