Microencapsulation Methods for Delivery of Protein Drugs
Microencapsulation Methods for Delivery of Protein Drugs
Microencapsulation Methods for Delivery of Protein Drugs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
224 Biotechnol. Bioprocess Eng. 2001, Vol. 6, No. 4<br />
Insulin was released with initial burst over<br />
achieved.<br />
first 30 minutes followed by a constant release up<br />
the<br />
to 3 h, after which the release rate declined.<br />
Limitations<br />
this method was initially proposed <strong>for</strong> en-<br />
Although<br />
encapsulations, it has some serious problems in<br />
zyme<br />
First, large w/o interface is produced during<br />
practice.<br />
reaction, where proteins or enzymes are likely to be<br />
the<br />
Second, large amounts <strong>of</strong> proteins may par-<br />
inactivated.<br />
in the polymerization reaction changing its bioticipate<br />
activity. Third, it is <strong>of</strong>ten hard to control the<br />
logical<br />
reaction. The yield and quality <strong>of</strong> the<br />
polymerization<br />
obtained by interfacial polymerization may<br />
membrane<br />
controlled by a number <strong>of</strong> factors, such as chemical<br />
be<br />
<strong>of</strong> reactive monomers, and reaction conditions.<br />
natures<br />
monomer concentrations, temperature, the mixing<br />
The<br />
and the reaction time are likely to be important<br />
rate,<br />
[105]. Fourth, exhaustive washing steps are<br />
parameters<br />
to remove monomers, byproducts, organic sol-<br />
required<br />
and surfactants. At the same time, several washvents,<br />
steps may lead to further loss <strong>of</strong> water soluble drugs.<br />
ing<br />
pH change due to HCl byproduct <strong>for</strong>med by reac-<br />
Fifth,<br />
<strong>of</strong> an acid chloride and an amine can damage the<br />
tion<br />
labile drugs.<br />
acid<br />
SUPERCRITICAL FLUID PRECIPITATION<br />
fluid is defined as a fluid <strong>of</strong> which tem-<br />
Supercritical<br />
and pressure are simultaneously higher than at<br />
perature<br />
critical point, i.e. critical temperature Tc and critical<br />
the<br />
Pc, at which the density <strong>of</strong> gas is equal to that<br />
pressure<br />
the remaining liquid and the surface between the<br />
<strong>of</strong><br />
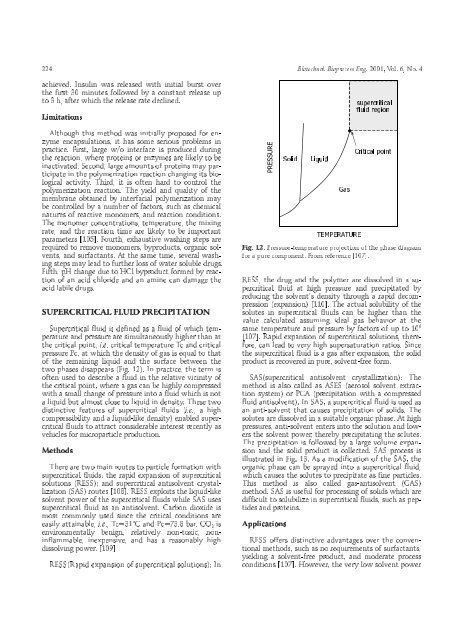
phases disappears (Fig. 12). In practice, the term is<br />
two<br />
used to describe a fluid in the relative vicinity <strong>of</strong><br />
<strong>of</strong>ten<br />
critical point, where a gas can be highly compressed<br />
the<br />
a small change <strong>of</strong> pressure into a fluid which is not<br />
with<br />
liquid but almost close to liquid in density. These two<br />
a<br />
features <strong>of</strong> supercritical fluids (i.e., a high<br />
distinctive<br />
and a liquid-like density) enabled super-<br />
compressibility<br />
fluids to attract considerable interest recently as<br />
critical<br />
<strong>for</strong> microparticle production.<br />
vehicles<br />
<strong>Methods</strong><br />
are two main routes to particle <strong>for</strong>mation with<br />
There<br />
fluids: the rapid expansion <strong>of</strong> supercritical<br />
supercritical<br />
(RESS); and supercritical antisolvent crystal-<br />
solutions<br />
(SAS) routes [108]. RESS exploits the liquid-like<br />
lization<br />
power <strong>of</strong> the supercritical fluids while SAS uses<br />
solvent<br />
fluid as an antisolvent. Carbon dioxide is<br />
supercritical<br />
commonly used since the critical conditions are<br />
most<br />
attainable, i.e., Tc=31°C and Pc=73.8 bar. CO2 is<br />
easily<br />
benign, relatively non-toxic, non-<br />
environmentally<br />
inexpensive, and has a reasonably high<br />
inflammable,<br />
dissolving power. [109]<br />
RESS(Rapid expansion <strong>of</strong> supercritical solutions): In<br />
12. Pressure-temperature projection <strong>of</strong> the phase diagram<br />
Fig.<br />
a pure component. From reference [107].<br />
<strong>for</strong><br />
the drug and the polymer are dissolved in a su-<br />
RESS,<br />
fluid at high pressure and precipitated by<br />
percritical<br />
the solvent’s density through a rapid decom-<br />
reducing<br />
(expansion) [110]. The actual solubility <strong>of</strong> the<br />
pression<br />
in supercritical fluids can be higher than the<br />
solutes<br />
calculated assuming ideal gas behavior at the<br />
value<br />
temperature and pressure by factors <strong>of</strong> up to 10 same 6<br />
Rapid expansion <strong>of</strong> supercritical solutions, there-<br />
[107].<br />
can lead to very high supersaturation ratios. Since<br />
<strong>for</strong>e,<br />
supercritical fluid is a gas after expansion, the solid<br />
the<br />
product is recovered in pure, solvent-free <strong>for</strong>m.<br />
antisolvent crystallization): The<br />
SAS(supercritical<br />
is also called as ASES (aerosol solvent extrac-<br />
method<br />
system) or PCA (precipitation with a compressed<br />
tion<br />
antisolvent). In SAS, a supercritical fluid is used as<br />
fluid<br />
anti-solvent that causes precipitation <strong>of</strong> solids. The<br />
an<br />
are dissolved in a suitable organic phase. At high<br />
solutes<br />
anti-solvent enters into the solution and low-<br />
pressures,<br />
the solvent power, thereby precipitating the solutes.<br />
ers<br />
precipitation is followed by a large volume expan-<br />
The<br />
and the solid product is collected. SAS process is<br />
sion<br />
in Fig. 13. As a modification <strong>of</strong> the SAS, the<br />
illustrated<br />
phase can be sprayed into a supercritical fluid,<br />
organic<br />
causes the solutes to precipitate as fine particles.<br />
which<br />
method is also called gas-antisolvent (GAS)<br />
This<br />
SAS is useful <strong>for</strong> processing <strong>of</strong> solids which are<br />
method.<br />
to solubilize in supercritical fluids, such as pep-<br />
difficult<br />
tides and proteins.<br />
Applications<br />
Solid Liquid<br />
Gas<br />
TEMPERATURE<br />
supercritical<br />
region<br />
fluid<br />
Critical point<br />
<strong>of</strong>fers distinctive advantages over the conven-<br />
RESS<br />
methods, such as no requirements <strong>of</strong> surfactants,<br />
tional<br />
a solvent-free product, and moderate process<br />
yielding<br />
[107]. However, the very low solvent power<br />
conditions