Effects of Dehydroepiandrosterone Therapy on Pubic Hair Growth ...
Effects of Dehydroepiandrosterone Therapy on Pubic Hair Growth ...
Effects of Dehydroepiandrosterone Therapy on Pubic Hair Growth ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1186 Binder et al. DHEA Replacement in Young Females J Clin Endocrinol Metab, April 2009, 94(4):1182–1190<br />
at inclusi<strong>on</strong>. In the placebo group, <strong>on</strong>e withdrawal was caused<br />
by relapse <str<strong>on</strong>g>of</str<strong>on</strong>g> craniopharyngioma, another due to the manifestati<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> type 1 diabetes mellitus. Two patients with placebo<br />
were lost at follow-up because <str<strong>on</strong>g>of</str<strong>on</strong>g> change in residence<br />
after 6 m<strong>on</strong>th therapy. The remaining 18 patients completed<br />
the trial.<br />
Recruitment was started in January 2004 and ended in March<br />
2006. All 23 patients attended the clinic at baseline after recruitment<br />
and randomizati<strong>on</strong>. There were 21 patients seen at the 6<br />
m<strong>on</strong>th visit, and 18 completed the trial at the 12 m<strong>on</strong>th visit. The<br />
last patient had her 12-m<strong>on</strong>th visit in April 2007.<br />
Study populati<strong>on</strong><br />
Baseline characteristics <str<strong>on</strong>g>of</str<strong>on</strong>g> the randomized study participants<br />
are shown in Table 1. The groups were well balanced<br />
with respect to age, weight, tumor and radiati<strong>on</strong> history, presence<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> additi<strong>on</strong>al pituitary horm<strong>on</strong>e deficiencies, doses <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
horm<strong>on</strong>es substituted, and pubic hair stage. All 23 patients<br />
had hypog<strong>on</strong>adotropic hypog<strong>on</strong>adism and were substituted<br />
with sex steroids. The <strong>on</strong>ly clinical characteristic that was<br />
different between the two groups was height (the placebo<br />
group was taller), which was presumably <str<strong>on</strong>g>of</str<strong>on</strong>g> no clinical relevance<br />
for this study.<br />
The analyses <str<strong>on</strong>g>of</str<strong>on</strong>g> the primary and sec<strong>on</strong>dary outcome measures<br />
involved all patients who took part in the 6-m<strong>on</strong>th visit and<br />
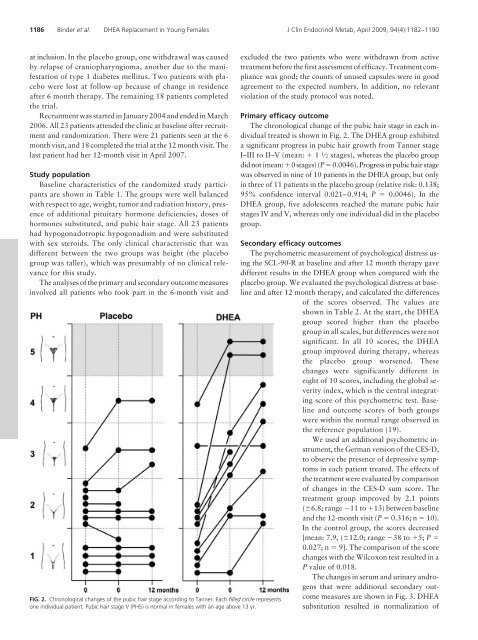
FIG. 2. Chr<strong>on</strong>ological changes <str<strong>on</strong>g>of</str<strong>on</strong>g> the pubic hair stage according to Tanner. Each filled circle represents<br />
<strong>on</strong>e individual patient. <strong>Pubic</strong> hair stage V (PH5) is normal in females with an age above 13 yr.<br />
excluded the two patients who were withdrawn from active<br />
treatment before the first assessment <str<strong>on</strong>g>of</str<strong>on</strong>g> efficacy. Treatment compliance<br />
was good; the counts <str<strong>on</strong>g>of</str<strong>on</strong>g> unused capsules were in good<br />
agreement to the expected numbers. In additi<strong>on</strong>, no relevant<br />
violati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the study protocol was noted.<br />
Primary efficacy outcome<br />
The chr<strong>on</strong>ological change <str<strong>on</strong>g>of</str<strong>on</strong>g> the pubic hair stage in each individual<br />
treated is shown in Fig. 2. The DHEA group exhibited<br />
a significant progress in pubic hair growth from Tanner stage<br />
I–III to II–V (mean: 1 1 ⁄2 stages), whereas the placebo group<br />
did not (mean: 0stages) (P0.0046). Progress in pubic hair stage<br />
was observed in nine <str<strong>on</strong>g>of</str<strong>on</strong>g> 10 patients in the DHEA group, but <strong>on</strong>ly<br />
in three <str<strong>on</strong>g>of</str<strong>on</strong>g> 11 patients in the placebo group (relative risk: 0.138;<br />
95% c<strong>on</strong>fidence interval 0.021–0.914; P 0.0046). In the<br />
DHEA group, five adolescents reached the mature pubic hair<br />
stages IV and V, whereas <strong>on</strong>ly <strong>on</strong>e individual did in the placebo<br />
group.<br />
Sec<strong>on</strong>dary efficacy outcomes<br />
The psychometric measurement <str<strong>on</strong>g>of</str<strong>on</strong>g> psychological distress using<br />
the SCL-90-R at baseline and after 12 m<strong>on</strong>th therapy gave<br />
different results in the DHEA group when compared with the<br />
placebo group. We evaluated the psychological distress at baseline<br />
and after 12 m<strong>on</strong>th therapy, and calculated the differences<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> the scores observed. The values are<br />
shown in Table 2. At the start, the DHEA<br />
group scored higher than the placebo<br />
group in all scales, but differences were not<br />
significant. In all 10 scores, the DHEA<br />
group improved during therapy, whereas<br />
the placebo group worsened. These<br />
changes were significantly different in<br />
eight <str<strong>on</strong>g>of</str<strong>on</strong>g> 10 scores, including the global severity<br />
index, which is the central integrating<br />
score <str<strong>on</strong>g>of</str<strong>on</strong>g> this psychometric test. Baseline<br />
and outcome scores <str<strong>on</strong>g>of</str<strong>on</strong>g> both groups<br />
were within the normal range observed in<br />
the reference populati<strong>on</strong> (19).<br />
We used an additi<strong>on</strong>al psychometric instrument,<br />
the German versi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the CES-D,<br />
to observe the presence <str<strong>on</strong>g>of</str<strong>on</strong>g> depressive symptoms<br />
in each patient treated. The effects <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
the treatment were evaluated by comparis<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> changes in the CES-D sum score. The<br />
treatment group improved by 2.1 points<br />
(6.8; range 11 to 13) between baseline<br />
and the 12-m<strong>on</strong>th visit (P 0.316; n 10).<br />
In the c<strong>on</strong>trol group, the scores decreased<br />
[mean: 7.9, (12.0; range 38 to 5; P <br />
0.027; n 9]. The comparis<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> the score<br />
changes with the Wilcox<strong>on</strong> test resulted in a<br />
P value <str<strong>on</strong>g>of</str<strong>on</strong>g> 0.018.<br />
The changes in serum and urinary androgens<br />
that were additi<strong>on</strong>al sec<strong>on</strong>dary out-<br />
come measures are shown in Fig. 3. DHEA<br />
substituti<strong>on</strong> resulted in normalizati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g>