PNP spectrochemistry Prelab assignment, Spring 2010 Page 1 PNP ...
PNP spectrochemistry Prelab assignment, Spring 2010 Page 1 PNP ...
PNP spectrochemistry Prelab assignment, Spring 2010 Page 1 PNP ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
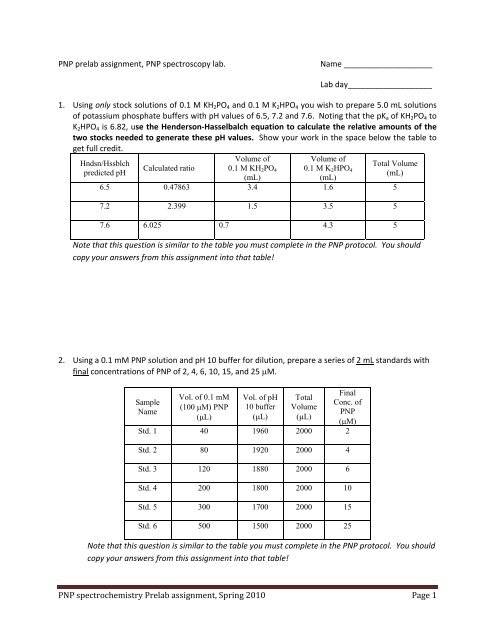
<strong>PNP</strong> prelab <strong>assignment</strong>, <strong>PNP</strong> spectroscopy lab. Name ____________________<br />
Lab day___________________<br />
1. Using only stock solutions of 0.1 M KH2PO4 and 0.1 M K2HPO4 you wish to prepare 5.0 mL solutions<br />
of potassium phosphate buffers with pH values of 6.5, 7.2 and 7.6. Noting that the pKa of KH2PO4 to<br />
K2HPO4 is 6.82, use the Henderson‐Hasselbalch equation to calculate the relative amounts of the<br />
two stocks needed to generate these pH values. Show your work in the space below the table to<br />
get full credit.<br />
Hndsn/Hssblch<br />
predicted pH<br />
6.5<br />
7.2<br />
7.6<br />
Calculated ratio<br />
Volume of<br />
0.1 M KH2PO4<br />
(mL)<br />
Volume of<br />
0.1 M K2HPO4<br />
(mL)<br />
Total Volume<br />
(mL)<br />
0.47863 3.4 1.6 5<br />
2.399 1.5 3.5 5<br />
6.025 0.7 4.3 5<br />
Note that this question is similar to the table you must complete in the <strong>PNP</strong> protocol. You should<br />
copy your answers from this <strong>assignment</strong> into that table!<br />
2. Using a 0.1 mM <strong>PNP</strong> solution and pH 10 buffer for dilution, prepare a series of 2 mL standards with<br />
final concentrations of <strong>PNP</strong> of 2, 4, 6, 10, 15, and 25 μM.<br />
Sample<br />
Name<br />
Std. 1<br />
Std. 2<br />
Std. 3<br />
Std. 4<br />
Std. 5<br />
Std. 6<br />
Vol. of 0.1 mM<br />
(100 μM) <strong>PNP</strong><br />
(μL)<br />
Vol. of pH<br />
10 buffer<br />
(μL)<br />
Total<br />
Volume<br />
(μL)<br />
Final<br />
Conc. of<br />
<strong>PNP</strong><br />
(μM)<br />
40 1960 2000 2<br />
80 1920 2000 4<br />
120 1880 2000 6<br />
200 1800 2000 10<br />
300 1700 2000 15<br />
500 1500 2000 25<br />
Note that this question is similar to the table you must complete in the <strong>PNP</strong> protocol. You should<br />
copy your answers from this <strong>assignment</strong> into that table!<br />
<strong>PNP</strong> <strong>spectrochemistry</strong> <strong>Prelab</strong> <strong>assignment</strong>, <strong>Spring</strong> <strong>2010</strong> <strong>Page</strong> 1
Sample calculation for problem 1<br />
For pH 6.5:<br />
<br />
<br />
6.5 6.82 <br />
<br />
0.32 <br />
<br />
0.47863 <br />
<br />
We have 2 variables, and 2 equations:<br />
Equation 1: Equation 2:<br />
A‐+HA = 5 mL A‐/HA = ratio (in this case 0.47863)<br />
Start:<br />
A‐/HA=0.47863<br />
A‐=0.47863HA<br />
Plug this into equation 1<br />
0.47863HA + HA = 5<br />
1.47863HA =5<br />
HA = 3.4<br />
Plug this into Equation 1:<br />
A‐+3.4 = 5.0<br />
A‐ = 1.6<br />
For problem 2, use M1V1 = M2V2 where M1=100 uM, M2=value in column 5, and V2=2000 uL. Solve for<br />
V1. Volume of pH 10 buffer is simply 2000 uL – V1.<br />
<strong>PNP</strong> <strong>spectrochemistry</strong> <strong>Prelab</strong> <strong>assignment</strong>, <strong>Spring</strong> <strong>2010</strong> <strong>Page</strong> 2