Chapter 7 LANGMUIR GEOL 414/514 CHEMICAL WEATHERING
Chapter 7 LANGMUIR GEOL 414/514 CHEMICAL WEATHERING
Chapter 7 LANGMUIR GEOL 414/514 CHEMICAL WEATHERING
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
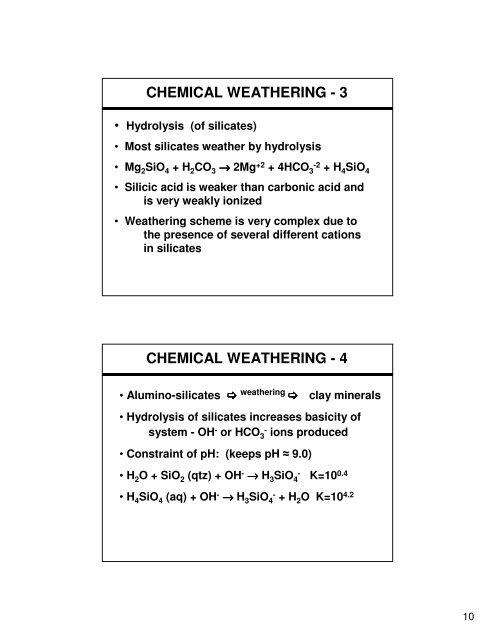
<strong>CHEMICAL</strong> <strong>WEATHERING</strong> - 3<br />
• Hydrolysis (of silicates)<br />
• Most silicates weather by hydrolysis<br />
• Mg 2SiO 4 + H 2CO 3 → 2Mg +2 + 4HCO 3 -2 + H4SiO 4<br />
• Silicic acid is weaker than carbonic acid and<br />
is very weakly ionized<br />
• Weathering scheme is very complex due to<br />
the presence of several different cations<br />
in silicates<br />
<strong>CHEMICAL</strong> <strong>WEATHERING</strong> - 4<br />
• Alumino-silicates weathering clay minerals<br />
• Hydrolysis of silicates increases basicity of<br />
system - OH - or HCO 3 - ions produced<br />
• Constraint of pH: (keeps pH 9.0)<br />
• H 2O + SiO 2 (qtz) + OH - → H 3SiO 4 - K=10 0.4<br />
• H 4SiO 4 (aq) + OH - → H 3SiO 4 - + H2O K=10 4.2<br />
10