A Study of the Chemical Degradation of ClONO2 in the ...
A Study of the Chemical Degradation of ClONO2 in the ...
A Study of the Chemical Degradation of ClONO2 in the ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
8 Ravishankara et al.: <strong>Chemical</strong> <strong>Degradation</strong><br />
3OO i<br />
I00<br />
00(p) + CIONO,<br />
i I I<br />
0 5 I0 15<br />
rntorr <strong>of</strong> ClONO 2<br />
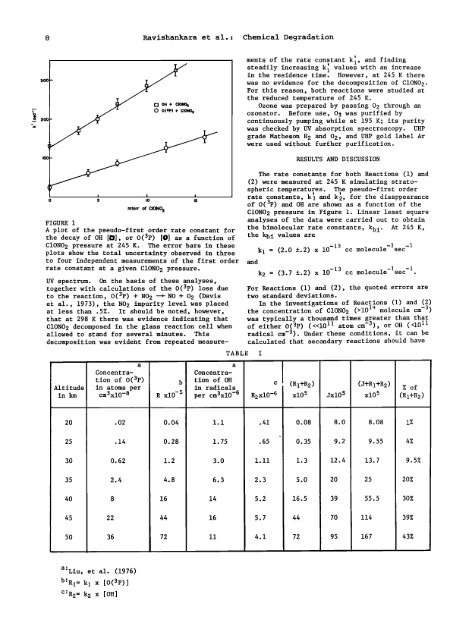
FIGURE 1<br />
A plot <strong>of</strong> <strong>the</strong> pseudo-first order rate constant for<br />
<strong>the</strong> decay <strong>of</strong> OH [O], or O(3p) [O] as a function <strong>of</strong><br />
ClONO<br />
!<br />
ments <strong>of</strong> <strong>the</strong> rate constant kl, and f<strong>in</strong>d<strong>in</strong>g<br />
steadily <strong>in</strong>creas<strong>in</strong>g k 1 values with an <strong>in</strong>crease<br />
<strong>in</strong> <strong>the</strong> residence time. However, at 245 K <strong>the</strong>re<br />
was no evidence for <strong>the</strong> decomposition <strong>of</strong> ClONO 2.<br />
For this reason, both reactions were studied at<br />
<strong>the</strong> reduced temperature <strong>of</strong> 245 K.<br />
Ozone was prepared by pass<strong>in</strong>g 02 through an<br />
ozonator. Before use, 03 was purified by<br />
cont<strong>in</strong>uously pump<strong>in</strong>g while at 195 K; its purity<br />
was checked by UV absorption spectroscopy. UHP<br />
grade Ma<strong>the</strong>son H 2 and 02, and UHP gold label Ar<br />
were used without fur<strong>the</strong>r purification.<br />
RESULTS AND DISCUSSION<br />
The rate constants for both Reactions (1) and<br />
(2) were measured at 245 K simulat<strong>in</strong>g stratospheric<br />
temperatures. The pseudo-first order<br />
rate constants, k' 1 and k2, ' for <strong>the</strong> disappearance<br />
<strong>of</strong> O(3p) and OH are shown as a function <strong>of</strong> <strong>the</strong><br />
ClON02 pressure <strong>in</strong> Figure 1. L<strong>in</strong>ear least square<br />
analyses <strong>of</strong> <strong>the</strong> data were carried out to obta<strong>in</strong><br />
<strong>the</strong> bimolecular rate constants, kbi. At 245 K,<br />
<strong>the</strong> kbi values are<br />
2 pressure at 245 K. The error bars <strong>in</strong> <strong>the</strong>se -13<br />
plots show <strong>the</strong> total uncerta<strong>in</strong>ty observed <strong>in</strong> three kl = (2.0 +.2) x 10<br />
to four <strong>in</strong>dependent measurements <strong>of</strong> <strong>the</strong> first order and<br />
-1 -1<br />
cc molecule sec<br />
rate constant at a given ClONO 2 pressure. k2 = (3.7 +.2) x 10 -13 cc molecule-lsec -1.<br />
UV spectrum. On <strong>the</strong> basis <strong>of</strong> <strong>the</strong>se analyses,<br />
toge<strong>the</strong>r with calculations <strong>of</strong> <strong>the</strong> O(3P) loss due For Reactions (1) and (2), <strong>the</strong> quoted errors are<br />
to <strong>the</strong> reaction, O(3P) + NO 2 --+ NO + 02 (Davis two standard deviations.<br />
et al., 1973), <strong>the</strong> NO 2 impurity level was placed<br />
at less than .5%. It should be noted, however,<br />
In <strong>the</strong> <strong>in</strong>vestigations <strong>of</strong> Reactions (1) and (2)<br />
<strong>the</strong> concentration <strong>of</strong> ClONO 2 (>1014 molecule cm -3)<br />
that at 298 K <strong>the</strong>re was evidence <strong>in</strong>dicat<strong>in</strong>g that was typically a thousand times greater than that<br />
ClONO 2 decomposed <strong>in</strong> <strong>the</strong> glass reaction cell when <strong>of</strong> ei<strong>the</strong>r O(3P) (