Three-Dimensional Aromaticity in Polyhedral Boranes and Related ...
Three-Dimensional Aromaticity in Polyhedral Boranes and Related ...
Three-Dimensional Aromaticity in Polyhedral Boranes and Related ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1120 Chemical Reviews, 2001, Vol. 101, No. 5 K<strong>in</strong>g<br />
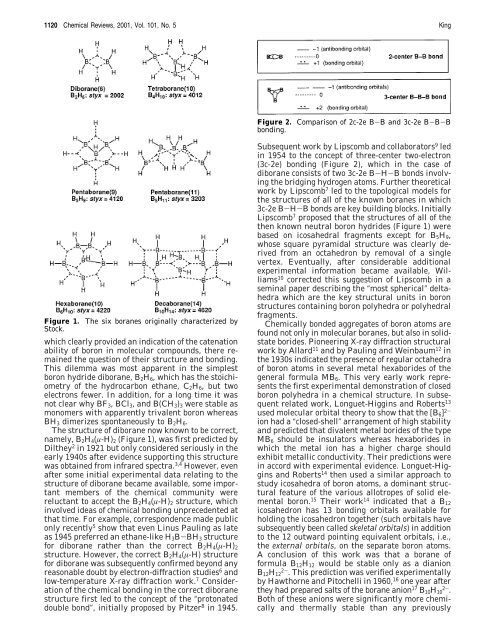
Figure 1. The six boranes orig<strong>in</strong>ally characterized by<br />
Stock.<br />
which clearly provided an <strong>in</strong>dication of the catenation<br />
ability of boron <strong>in</strong> molecular compounds, there rema<strong>in</strong>ed<br />
the question of their structure <strong>and</strong> bond<strong>in</strong>g.<br />
This dilemma was most apparent <strong>in</strong> the simplest<br />
boron hydride diborane, B2H6, which has the stoichiometry<br />
of the hydrocarbon ethane, C2H6, but two<br />
electrons fewer. In addition, for a long time it was<br />
not clear why BF3, BCl3, <strong>and</strong> B(CH3)3 were stable as<br />
monomers with apparently trivalent boron whereas<br />
BH3 dimerizes spontaneously to B2H6.<br />
The structure of diborane now known to be correct,<br />
namely, B2H4(µ-H)2 (Figure 1), was first predicted by<br />
Dilthey 2 <strong>in</strong> 1921 but only considered seriously <strong>in</strong> the<br />
early 1940s after evidence support<strong>in</strong>g this structure<br />
was obta<strong>in</strong>ed from <strong>in</strong>frared spectra. 3,4 However, even<br />
after some <strong>in</strong>itial experimental data relat<strong>in</strong>g to the<br />
structure of diborane became available, some important<br />
members of the chemical community were<br />
reluctant to accept the B2H4(µ-H)2 structure, which<br />
<strong>in</strong>volved ideas of chemical bond<strong>in</strong>g unprecedented at<br />
that time. For example, correspondence made public<br />
only recently 5 show that even L<strong>in</strong>us Paul<strong>in</strong>g as late<br />
as 1945 preferred an ethane-like H3B-BH3 structure<br />
for diborane rather than the correct B2H4(µ-H)2<br />
structure. However, the correct B2H4(µ-H) structure<br />
for diborane was subsequently confirmed beyond any<br />
reasonable doubt by electron-diffraction studies 6 <strong>and</strong><br />
low-temperature X-ray diffraction work. 7 Consideration<br />
of the chemical bond<strong>in</strong>g <strong>in</strong> the correct diborane<br />
structure first led to the concept of the “protonated<br />
double bond”, <strong>in</strong>itially proposed by Pitzer 8 <strong>in</strong> 1945.<br />
Figure 2. Comparison of 2c-2e B-B <strong>and</strong> 3c-2e B-B-B<br />
bond<strong>in</strong>g.<br />
Subsequent work by Lipscomb <strong>and</strong> collaborators 9 led<br />
<strong>in</strong> 1954 to the concept of three-center two-electron<br />
(3c-2e) bond<strong>in</strong>g (Figure 2), which <strong>in</strong> the case of<br />
diborane consists of two 3c-2e B-H-B bonds <strong>in</strong>volv<strong>in</strong>g<br />
the bridg<strong>in</strong>g hydrogen atoms. Further theoretical<br />
work by Lipscomb 7 led to the topological models for<br />
the structures of all of the known boranes <strong>in</strong> which<br />
3c-2e B-H-B bonds are key build<strong>in</strong>g blocks. Initially<br />
Lipscomb 7 proposed that the structures of all of the<br />
then known neutral boron hydrides (Figure 1) were<br />
based on icosahedral fragments except for B5H9,<br />
whose square pyramidal structure was clearly derived<br />
from an octahedron by removal of a s<strong>in</strong>gle<br />
vertex. Eventually, after considerable additional<br />
experimental <strong>in</strong>formation became available, Williams<br />
10 corrected this suggestion of Lipscomb <strong>in</strong> a<br />
sem<strong>in</strong>al paper describ<strong>in</strong>g the “most spherical” deltahedra<br />
which are the key structural units <strong>in</strong> boron<br />
structures conta<strong>in</strong><strong>in</strong>g boron polyhedra or polyhedral<br />
fragments.<br />
Chemically bonded aggregates of boron atoms are<br />
found not only <strong>in</strong> molecular boranes, but also <strong>in</strong> solidstate<br />
borides. Pioneer<strong>in</strong>g X-ray diffraction structural<br />
work by Allard 11 <strong>and</strong> by Paul<strong>in</strong>g <strong>and</strong> We<strong>in</strong>baum 12 <strong>in</strong><br />
the 1930s <strong>in</strong>dicated the presence of regular octahedra<br />
of boron atoms <strong>in</strong> several metal hexaborides of the<br />
general formula MB6. This very early work represents<br />
the first experimental demonstration of closed<br />
boron polyhedra <strong>in</strong> a chemical structure. In subsequent<br />
related work, Longuet-Higg<strong>in</strong>s <strong>and</strong> Roberts 13<br />
used molecular orbital theory to show that the [B6] 2ion<br />
had a “closed-shell” arrangement of high stability<br />
<strong>and</strong> predicted that divalent metal borides of the type<br />
MB6 should be <strong>in</strong>sulators whereas hexaborides <strong>in</strong><br />
which the metal ion has a higher charge should<br />
exhibit metallic conductivity. Their predictions were<br />
<strong>in</strong> accord with experimental evidence. Longuet-Higg<strong>in</strong>s<br />
<strong>and</strong> Roberts 14 then used a similar approach to<br />
study icosahedra of boron atoms, a dom<strong>in</strong>ant structural<br />
feature of the various allotropes of solid elemental<br />
boron. 15 Their work 14 <strong>in</strong>dicated that a B12<br />
icosahedron has 13 bond<strong>in</strong>g orbitals available for<br />
hold<strong>in</strong>g the icosahedron together (such orbitals have<br />
subsequently been called skeletal orbitals) <strong>in</strong> addition<br />
to the 12 outward po<strong>in</strong>t<strong>in</strong>g equivalent orbitals, i.e.,<br />
the external orbitals, on the separate boron atoms.<br />
A conclusion of this work was that a borane of<br />
formula B12H12 would be stable only as a dianion<br />
B12H12 2- . This prediction was verified experimentally<br />
by Hawthorne <strong>and</strong> Pitochelli <strong>in</strong> 1960, 16 one year after<br />
they had prepared salts of the borane anion 17 B10H10 2- .<br />
Both of these anions were significantly more chemically<br />
<strong>and</strong> thermally stable than any previously