Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
C.A. Miller and K.H. Raney/Colloids Surfaces A: Physicochem. Eng. Aspects 74 (1993) 169-215 189<br />
micro emulsion formed between the L1 and oil<br />
phases. At 48 º C, a similar phase progression<br />
was observed, with a middle-phase microemulsion<br />
forming in place <strong>of</strong> the oil-in-water<br />
microemulsion. As shown in Fig. 24, plots <strong>of</strong><br />
the position <strong>of</strong> the oil-micro emulsion interface<br />
versus the square root <strong>of</strong> time were straight lines<br />
in both cases indicating diffusion-controlled<br />
mass transfer. In Fig. 24, an arbitrary constant<br />
has been added to each position for ease <strong>of</strong><br />
comparison, i.e. x = 0 is not the initial contact<br />
position. At any given time, the relative slopes<br />
<strong>of</strong> the two lines are indicative <strong>of</strong> the relative<br />
rates <strong>of</strong> oil solubilization. In this case, the rate <strong>of</strong><br />
solubilization near the PIT is 2.2 times greater<br />
than at 40ºC.<br />
Well above the phase inversion temperatures<br />
for both hexadecane and tetradecane, 1 wt.%<br />
C12E5 exists as a dispersion <strong>of</strong> liquid crystal Lα<br />
in water. Rapid movement <strong>of</strong> the liquid crystal<br />
to the oil occurred upon contacting, causing<br />
extensive spontaneous <strong>emulsification</strong> <strong>of</strong> water in<br />
the oil phase. Eventually a layer depleted in<br />
liquid crystal formed near the oil interface.<br />
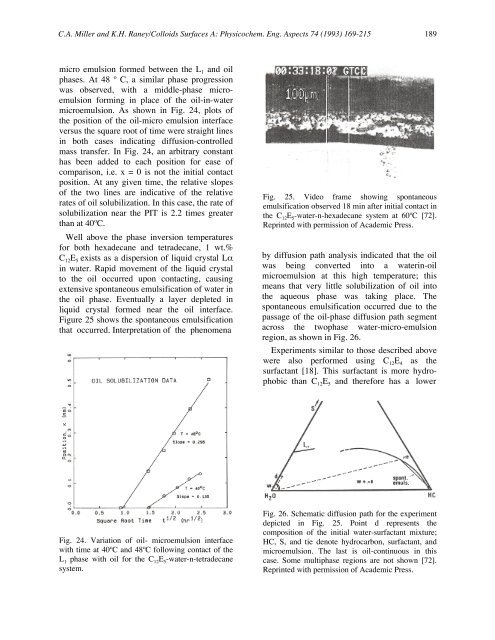
Figure 25 shows the spontaneous <strong>emulsification</strong><br />
that occurred. Interpretation <strong>of</strong> the phenomena<br />
Fig. 24. Variation <strong>of</strong> oil- microemulsion interface<br />
with time at 40ºC and 48ºC following contact <strong>of</strong> the<br />
L 1 phase with oil for the C 12E 5-water-n-tetradecane<br />
system.<br />
Fig. 25. Video frame showing spontaneous<br />
<strong>emulsification</strong> observed 18 min after initial contact in<br />
the C 12E 5-water-n-hexadecane system at 60ºC [72].<br />
Reprinted with permission <strong>of</strong> Academic Press.<br />
by diffusion path analysis indicated that the oil<br />
was being converted into a waterin-oil<br />
microemulsion at this high temperature; this<br />
means that very little solubilization <strong>of</strong> oil into<br />
the aqueous phase was taking place. The<br />
spontaneous <strong>emulsification</strong> occurred due to the<br />
passage <strong>of</strong> the oil-phase diffusion path segment<br />
across the twophase water-micro-emulsion<br />
region, as shown in Fig. 26.<br />
Experiments similar to those described above<br />
were also performed using C12E4 as the<br />
surfactant [18]. This surfactant is more hydrophobic<br />
than C12E5 and therefore has a lower<br />
Fig. 26. Schematic diffusion path for the experiment<br />
depicted in Fig. 25. Point d represents the<br />
composition <strong>of</strong> the initial water-surfactant mixture;<br />
HC, S, and tie denote hydrocarbon, surfactant, and<br />
microemulsion. The last is oil-continuous in this<br />
case. Some multiphase regions are not shown [72].<br />
Reprinted with permission <strong>of</strong> Academic Press.