Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
C.A. Miller and K.H. Raney/Colloids Surfaces A: Physicochem. Eng. Aspects 74 (1993) 169-215 205<br />
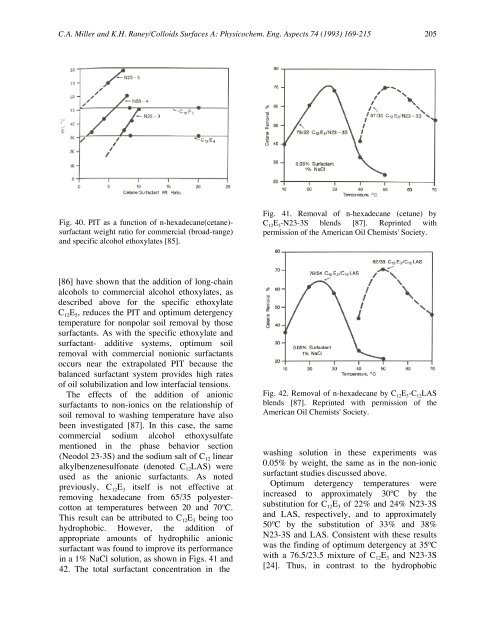
Fig. 40. PIT as a function <strong>of</strong> n-hexadecane(cetane)surfactant<br />
weight ratio for commercial (broad-range)<br />
and specific alcohol ethoxylates [85].<br />
[86] have shown that the addition <strong>of</strong> long-chain<br />
alcohols to commercial alcohol ethoxylates, as<br />
described above for the specific ethoxylate<br />
C 12E 5, reduces the PIT and optimum <strong>detergency</strong><br />
temperature for nonpolar soil removal by those<br />
surfactants. As with the specific ethoxylate and<br />
surfactant- additive systems, optimum soil<br />
removal with commercial nonionic surfactants<br />
occurs near the extrapolated PIT because the<br />
balanced surfactant system provides high rates<br />
<strong>of</strong> oil solubilization and low interfacial tensions.<br />
The effects <strong>of</strong> the addition <strong>of</strong> anionic<br />
surfactants to non-ionics on the relationship <strong>of</strong><br />
soil removal to washing temperature have also<br />
been investigated [87]. In this case, the same<br />
commercial sodium alcohol ethoxysulfate<br />
mentioned in the phase behavior section<br />
(Neodol 23-3S) and the sodium salt <strong>of</strong> C 12 linear<br />
alkylbenzenesulfonate (denoted C 12LAS) were<br />
used as the anionic surfactants. As noted<br />
previously, C 12E 3 itself is not effective at<br />
removing hexadecane from 65/35 polyestercotton<br />
at temperatures between 20 and 70ºC.<br />
This result can be attributed to C 12E 3 being too<br />
hydrophobic. However, the addition <strong>of</strong><br />
appropriate amounts <strong>of</strong> hydrophilic anionic<br />
surfactant was found to improve its performance<br />
in a 1% NaCl solution, as shown in Figs. 41 and<br />
42. The total surfactant concentration in the<br />
Fig. 41. Removal <strong>of</strong> n-hexadecane (cetane) by<br />
C 12E 3-N23-3S blends [87]. Reprinted with<br />
permission <strong>of</strong> the American Oil Chemists' Society.<br />
Fig. 42. Removal <strong>of</strong> n-hexadecane by C 12E 3-C 12LAS<br />
blends [87]. Reprinted with permission <strong>of</strong> the<br />
American Oil Chemists' Society.<br />
washing solution in these experiments was<br />
0.05% by weight, the same as in the non-ionic<br />
surfactant studies discussed above.<br />
Optimum <strong>detergency</strong> temperatures were<br />
increased to approximately 30ºC by the<br />
substitution for C 12E 3 <strong>of</strong> 22% and 24% N23-3S<br />
and LAS, respectively, and to approximately<br />
50ºC by the substitution <strong>of</strong> 33% and 38%<br />
N23-3S and LAS. Consistent with these results<br />
was the finding <strong>of</strong> optimum <strong>detergency</strong> at 35ºC<br />
with a 76.5/23.5 mixture <strong>of</strong> C 12E 3 and N23-3S<br />
[24]. Thus, in contrast to the hydrophobic