Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
Solubilization-emulsification mechanisms of detergency
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
C.A. Miller and K.H. Raney/Colloids Surfaces A: Physicochem. Eng. Aspects 74 (1993) 169-215 207<br />
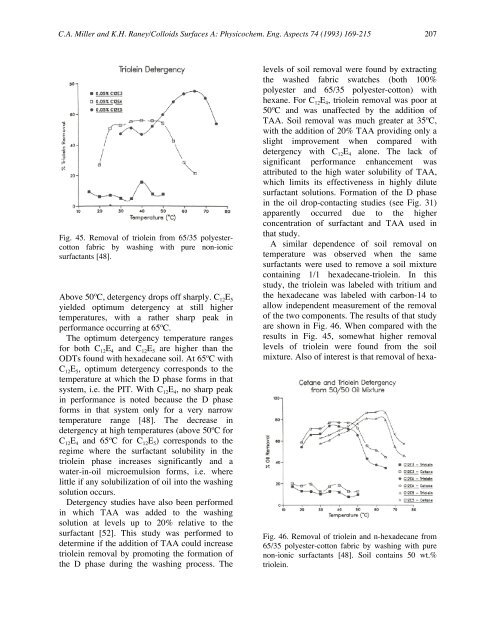
Fig. 45. Removal <strong>of</strong> triolein from 65/35 polyestercotton<br />
fabric by washing with pure non-ionic<br />
surfactants [48].<br />
Above 50ºC, <strong>detergency</strong> drops <strong>of</strong>f sharply. C 12E 5<br />
yielded optimum <strong>detergency</strong> at still higher<br />
temperatures, with a rather sharp peak in<br />
performance occurring at 65ºC.<br />
The optimum <strong>detergency</strong> temperature ranges<br />
for both C 12E 4 and C 12E 5 are higher than the<br />
ODTs found with hexadecane soil. At 65ºC with<br />
C 12E 5, optimum <strong>detergency</strong> corresponds to the<br />
temperature at which the D phase forms in that<br />
system, i.e. the PIT. With C 12E 4, no sharp peak<br />
in performance is noted because the D phase<br />
forms in that system only for a very narrow<br />
temperature range [48]. The decrease in<br />
<strong>detergency</strong> at high temperatures (above 50ºC for<br />
C 12E 4 and 65ºC for C 12E 5) corresponds to the<br />
regime where the surfactant solubility in the<br />
triolein phase increases significantly and a<br />
water-in-oil microemulsion forms, i.e. where<br />
little if any solubilization <strong>of</strong> oil into the washing<br />
solution occurs.<br />
Detergency studies have also been performed<br />
in which TAA was added to the washing<br />
solution at levels up to 20% relative to the<br />
surfactant [52]. This study was performed to<br />
determine if the addition <strong>of</strong> TAA could increase<br />
triolein removal by promoting the formation <strong>of</strong><br />
the D phase during the washing process. The<br />
levels <strong>of</strong> soil removal were found by extracting<br />
the washed fabric swatches (both 100%<br />
polyester and 65/35 polyester-cotton) with<br />
hexane. For C 12E 4, triolein removal was poor at<br />
50ºC and was unaffected by the addition <strong>of</strong><br />
TAA. Soil removal was much greater at 35ºC,<br />
with the addition <strong>of</strong> 20% TAA providing only a<br />
slight improvement when compared with<br />
<strong>detergency</strong> with C 12E 4 alone. The lack <strong>of</strong><br />
significant performance enhancement was<br />
attributed to the high water solubility <strong>of</strong> TAA,<br />
which limits its effectiveness in highly dilute<br />
surfactant solutions. Formation <strong>of</strong> the D phase<br />
in the oil drop-contacting studies (see Fig. 31)<br />
apparently occurred due to the higher<br />
concentration <strong>of</strong> surfactant and TAA used in<br />
that study.<br />
A similar dependence <strong>of</strong> soil removal on<br />
temperature was observed when the same<br />
surfactants were used to remove a soil mixture<br />
containing 1/1 hexadecane-triolein. In this<br />
study, the triolein was labeled with tritium and<br />
the hexadecane was labeled with carbon-14 to<br />
allow independent measurement <strong>of</strong> the removal<br />
<strong>of</strong> the two components. The results <strong>of</strong> that study<br />
are shown in Fig. 46. When compared with the<br />
results in Fig. 45, somewhat higher removal<br />
levels <strong>of</strong> triolein were found from the soil<br />
mixture. Also <strong>of</strong> interest is that removal <strong>of</strong> hexa-<br />
Fig. 46. Removal <strong>of</strong> triolein and n-hexadecane from<br />
65/35 polyester-cotton fabric by washing with pure<br />
non-ionic surfactants [48]. Soil contains 50 wt.%<br />
triolein.