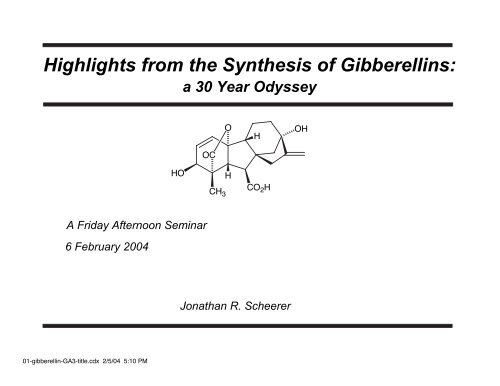
Highlights from the Synthesis of Gibberellins:
Highlights from the Synthesis of Gibberellins:
Highlights from the Synthesis of Gibberellins:
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Highlights</strong> <strong>from</strong> <strong>the</strong> Syn<strong>the</strong>sis <strong>of</strong> <strong>Gibberellins</strong>:<br />
a 30 Year Odyssey<br />
HO<br />
A Friday Afternoon Seminar<br />
6 February 2004<br />
01-gibberellin-GA3-title.cdx 2/5/04 5:10 PM<br />
OC<br />
CH 3<br />
O OH<br />
H<br />
H<br />
CO 2H<br />
Jonathan R. Scheerer
<strong>Highlights</strong> <strong>from</strong> <strong>the</strong> Syn<strong>the</strong>sis <strong>of</strong> <strong>Gibberellins</strong>:<br />
HO<br />
OC<br />
CH 3<br />
O OH<br />
H<br />
H<br />
CO 2H<br />
Outline <strong>of</strong> Presentation:<br />
I. Introduction to <strong>Gibberellins</strong>: History, Ubiquity, and Biology<br />
II. Biosyn<strong>the</strong>sis<br />
III. Gibberellic Acid: Structure and Reactivity<br />
IV. Conversion <strong>of</strong> Gibberellic Acid into o<strong>the</strong>r <strong>Gibberellins</strong><br />
V. Total Syn<strong>the</strong>sis<br />
VI. Partial Syn<strong>the</strong>sis / Stragedy<br />
Relvant Reviews:<br />
Mander, Chem. Rev. 1992, 573-612.<br />
Mander, Nat. Prod. Rep. 2003, 49-69.<br />
MacMillan, Nat. Prod. Rep. 1996, 229.<br />
Crozier, A. Ed. The Biochemistry and Physiology <strong>of</strong> <strong>Gibberellins</strong>. Praeger: New York, 1983. (vol 1 and 2)<br />
02-gibberellin-outline.cdx 2/5/04 5:11 PM
HO<br />
OC<br />
CH 3<br />
O<br />
H<br />
A Brief History <strong>of</strong> Gibberellin Research:<br />
H<br />
CO 2H<br />
1898 - first research paper, links disease to fungal infection<br />
OH<br />
1828 - first reports <strong>of</strong> "bakanae" disease in rice plants (foolish seedling; stupid rice crop)<br />
1912 - Kurosawa found that filtrates <strong>from</strong> infected dried rice seedlings also causes disease<br />
Concludes that bakanae is caused by discrete chemical<br />
1935 - First use <strong>of</strong> term "gibberellin" in scientific literature<br />
1938 - Crystalline compound (mix <strong>of</strong> three gibberellins) isolated <strong>from</strong> fungal filtrate<br />
1945 - Research expands to U.S. and U.K.<br />
1955 - compound isolated. termed "gibberellic acid"<br />
1958 - correct structure proposed (stereochemical ambiguities remain)<br />
1961 - structure verified by X-ray<br />
1978 - First total syn<strong>the</strong>sis (Corey)<br />
03-bakanae.cdx 2/5/04 5:19 PM
2<br />
HO2C 19<br />
C 20 and C 19 <strong>Gibberellins</strong>: Structure and Nomenclature<br />
1<br />
A<br />
3 4<br />
2<br />
3<br />
20<br />
CH3 10<br />
H<br />
CH3 18<br />
OC<br />
19<br />
CH3 18<br />
O<br />
H<br />
5<br />
9<br />
6<br />
H C<br />
8 14<br />
7 CO2H B<br />
11<br />
H<br />
7 CO2H 12<br />
15<br />
D<br />
13<br />
16<br />
C 20-Gibberellin Skeleton<br />
1<br />
5<br />
9<br />
6<br />
11<br />
8<br />
12<br />
14<br />
15<br />
13<br />
16<br />
C 19-Gibberellin Skeleton<br />
05-gibberellin-structureB.cdx 2/5/04 5:13 PM<br />
17<br />
17<br />
GA 12<br />
HO2C HO2C ent-gibberell-16-ene-7,19-dioic acid<br />
GA 9<br />
O<br />
R<br />
H<br />
O<br />
H<br />
R = CO 2H<br />
ent-norgibberell-16-ene-7,19-dioic acid 19,10-lactone
HO<br />
2<br />
3<br />
11 12<br />
1 O<br />
OC<br />
19 5<br />
H<br />
9<br />
6<br />
H<br />
8 14<br />
15<br />
OH<br />
13<br />
16<br />
17<br />
CH3 18<br />
7 CO2H Gibberellic Acid (GA 3)<br />
H 3C<br />
Fermented <strong>from</strong> Gibberellia fujikuroi (a fungus) on ton scale<br />
Bioactive at low concentrations ((sub-nanomolar common for applications)<br />
Widely investigated and applied for commercial uses<br />
Retail prices: $10 / g<br />
Current yields: 15-30 g / L culture<br />
Also bio-available in decent quantity:<br />
07-gibberellin-GA3.cdx 2/5/04 8:55 PM<br />
GA 3<br />
HO<br />
OH<br />
O<br />
OC<br />
R<br />
O<br />
H<br />
H<br />
Me<br />
GA 4<br />
O<br />
H<br />
H<br />
CO 2H<br />
OH<br />
R = CO2H HO<br />
OC<br />
O<br />
H<br />
Me<br />
H<br />
CO 2H<br />
GA 1<br />
OH
HO<br />
OC<br />
CH 3<br />
O OH<br />
H<br />
H<br />
CO 2H<br />
Representative Biological Functions <strong>of</strong> <strong>Gibberellins</strong>:<br />
A Brief History <strong>of</strong> <strong>Gibberellins</strong>:<br />
– Stimulate stem elongation by stimulating cell division elongation<br />
– Breaks seed dormancy in plants which require winter freezing<br />
– Stimulates flowering/budding in response to leng<strong>the</strong>ning days<br />
– Can induce seedless fruit development (par<strong>the</strong>nocarpic)<br />
– Can delay senescence (ripening) in leaves and fruit<br />
– Induces maleness (sex expression) in dioecious flowers<br />
– O<strong>the</strong>r growth effects on fruit and budding<br />
08-gibberellin-functions.cdx 2/5/04 5:15 PM
HO 2C<br />
HO Me<br />
OH<br />
mevalonic acid (MVA)<br />
10-gibberellin-biosyn<strong>the</strong>sis1cdx 2/5/04 12:29 PM<br />
Gibberellin Biosyn<strong>the</strong>sis: Three Stages<br />
H3C H<br />
Stage A Stage B<br />
OC<br />
O<br />
H<br />
CH 3<br />
H<br />
H3C CH3 ent-kaur-16-ene<br />
H<br />
CO 2H<br />
C 19-gibberellins<br />
and C 20-gibberellins<br />
HO 2C<br />
H 3C<br />
H<br />
CH 3<br />
H<br />
CO 2H<br />
HO 2C<br />
Stage C<br />
H 3C<br />
H<br />
CH 3<br />
H<br />
CHO<br />
GA 12-aldehyde
HO 2C<br />
HO<br />
*<br />
CH 3<br />
OH<br />
mevalonic acid (MVA)<br />
DMAPP<br />
Dimethylallyl<br />
pyrophosphate<br />
OPP IPP<br />
Isopentenyl<br />
pyrophosphate<br />
OPP<br />
11-gibberellin-biosyn<strong>the</strong>sis2 2/5/04 12:30 PM<br />
Gibberellin Biosyn<strong>the</strong>sis: Stage A<br />
Stage A<br />
H 3C<br />
H<br />
H3C CH3 IPP IPP<br />
OPP<br />
GPP<br />
*<br />
*<br />
H<br />
*<br />
*<br />
ent-kaur-16-ene<br />
FPP<br />
H 3C<br />
H<br />
H 3C CH 3<br />
H<br />
OPP<br />
OPP<br />
ent-CCP<br />
ent-copalyl<br />
pyrophosphate<br />
OPP<br />
GGPP<br />
geranyl-geranyl<br />
pyrophosphate
H 3C<br />
H<br />
H 3C CH 3<br />
12-biosyn<strong>the</strong>sis3.cdx 2/5/04 10:11 PM<br />
H<br />
Gibberellin Biosyn<strong>the</strong>sis: ent-CCP to ent-kaurene<br />
OPP<br />
ent-CCP<br />
ent-copalyl pyrophosphate<br />
H a<br />
H<br />
H 5S<br />
H 5R<br />
H 4<br />
OPP<br />
H 3C<br />
H<br />
H 3C CH 3<br />
H<br />
ent-kaur-16-ene<br />
H5R H5S H a<br />
H<br />
H 4<br />
H 5R<br />
H 5S<br />
H<br />
H5R H5S H a<br />
H a<br />
H<br />
H 4<br />
H
H S<br />
H R<br />
H 3C<br />
H<br />
H 3C CH 3<br />
H 3C<br />
OH<br />
H<br />
CH3 13-biosyn<strong>the</strong>sis4.cdx 2/5/04 10:35 PM<br />
H<br />
ent-kaur-16-ene<br />
H<br />
H 3C<br />
H<br />
H<br />
HS CH3 O<br />
ent-kaur-16-en-19-al<br />
Gibberellin Biosyn<strong>the</strong>sis: Stage B<br />
Stage B<br />
ent-kaur-16-en-19-ol<br />
HO 2C<br />
CH 3 H<br />
H<br />
CH 3<br />
CHO<br />
GA 12-aldehyde<br />
biosyn<strong>the</strong>tic progenitor<br />
<strong>of</strong> all gibberellins<br />
same biosyn<strong>the</strong>sis for<br />
fungal or higher order plants<br />
H 3C<br />
H<br />
HO 2C CH 3<br />
H a<br />
H<br />
Hd Hb H c<br />
HO 2C<br />
H<br />
H 3C<br />
H<br />
HO 2C CH 3<br />
CH 3 H<br />
H b<br />
H a<br />
H<br />
H d<br />
Hd Hb O<br />
see next slide<br />
OH
H<br />
HO2C Enz<br />
H 3C<br />
H 3C CH 3<br />
O<br />
H<br />
H<br />
H<br />
ent-kaur-16-ene<br />
Fe 4+<br />
OH<br />
14-biosyn<strong>the</strong>sis5.cdx 2/5/04 12:37 PM<br />
H<br />
Gibberellin Biosyn<strong>the</strong>sis: Ring Contraction<br />
Enz<br />
HO 2C<br />
Stage B<br />
OH<br />
Fe 3+<br />
HO<br />
HO2C H<br />
H<br />
O<br />
O<br />
H<br />
H<br />
H<br />
H<br />
1,2-radical<br />
shift<br />
HO 2C<br />
CH 3 H<br />
H<br />
CH 3<br />
HO 2C OHC<br />
H<br />
radical trapping<br />
CHO<br />
GA 12-aldehyde
HO 2C<br />
CH 3 H<br />
H<br />
CH 3<br />
CHO<br />
GA 12-aldehyde<br />
R<br />
O<br />
HO 2C<br />
CO<br />
OH<br />
-very complex<br />
H<br />
-parallel pathways<br />
Me<br />
-organism dependent<br />
(fungal or higher order plants)<br />
-converge to common GA<br />
GA n<br />
R<br />
OC<br />
Me<br />
CH 3 H<br />
H<br />
CH 3<br />
H<br />
CO 2H<br />
CO 2H<br />
many complex, as yet incompletely<br />
defined, oxidative processes<br />
15-biosyn<strong>the</strong>sis6.cdx 2/5/04 12:40 PM<br />
Gibberellin Biosyn<strong>the</strong>sis: Stage C<br />
O<br />
H<br />
H<br />
CO 2H<br />
R<br />
R<br />
and/or<br />
HO<br />
R<br />
HO 2C<br />
O<br />
R<br />
HO2C CO<br />
Me<br />
CH 3 H<br />
H<br />
CH 3<br />
H<br />
H<br />
CH 3<br />
CHO<br />
early or late oxidations <strong>of</strong> C3, C13<br />
H<br />
OH H<br />
CO 2H<br />
CO 2H<br />
R<br />
R<br />
OH<br />
R<br />
HO 2C<br />
HO<br />
R<br />
HO2C R<br />
HO2C CH 3 H<br />
H<br />
CH 3<br />
R = H, OH<br />
O<br />
H<br />
CH 3<br />
H<br />
CH 3<br />
oxidative decarboxlyation<br />
CO 2H<br />
H<br />
CO 2H<br />
H<br />
CO 2H<br />
R<br />
R<br />
R
HO<br />
GA 3<br />
OC<br />
CH 3<br />
O OH<br />
H<br />
H<br />
Gibberellic Acid<br />
HO<br />
OC<br />
CH 3<br />
H<br />
retrograde<br />
aldol / aldol<br />
Rearrangements <strong>of</strong> Gibberellic Acid in Basic Media<br />
CO 2H<br />
CO 2H<br />
via<br />
O<br />
O OH<br />
H<br />
16-GA3rearrangements.cdx 2/5/04 11:41 AM<br />
O<br />
–OOC<br />
–O<br />
H 3C<br />
H<br />
H<br />
CH 3<br />
Base<br />
H<br />
CO 2H<br />
CO 2H<br />
0.01 N NaOH<br />
O OH<br />
H<br />
OH<br />
HO<br />
HO<br />
OC<br />
O<br />
CH 3<br />
H<br />
CO<br />
HO<br />
HO<br />
CH 3<br />
H<br />
HO 2C<br />
CO 2H<br />
H<br />
CO 2H<br />
isolable<br />
H<br />
CH 3<br />
O OH<br />
H<br />
favored equatorial<br />
C3 configuration<br />
H<br />
CO 2H<br />
OH<br />
OH<br />
transformation can be<br />
effected by palladium
CH 3<br />
HO<br />
GA 3<br />
H<br />
OC<br />
CO 2H<br />
Rearrangements on Gibberellic Acid in Acidic Media<br />
CH 3<br />
O OH<br />
H<br />
H<br />
Gibberellic Acid<br />
H<br />
CO 2H<br />
CH 3<br />
O<br />
1,2 shift<br />
OH<br />
17-GA3rearrangements-acid.cdx 2/5/04 11:38 AM<br />
R<br />
CH 3<br />
H<br />
H +<br />
(or H 2NNH 2)<br />
CO 2H<br />
OH<br />
CH 3<br />
HO<br />
HO 2C<br />
CH 3<br />
Thermodynamically<br />
more stable C9 epimer<br />
H<br />
CH 3<br />
H<br />
CO 2H<br />
Gibberellenic Acid<br />
CO 2H<br />
"...gibberellic acid has enjoyed<br />
a significant notoriety for instability and<br />
rearrangement. This view appears to be<br />
exagerated." L.Mander<br />
OH<br />
OH<br />
CH 3<br />
H +<br />
H<br />
H +<br />
CO 2H<br />
H 2NNH 2<br />
OH
AcO<br />
OC<br />
Me<br />
O<br />
H<br />
CO 2Me<br />
BH 3<br />
18-GA-C11oxidation.cdx 2/5/04 11:34 AM<br />
C11 oxidation: Bishydroboration<br />
H 2B<br />
BH 3•SMe 2<br />
H 2O 2, NaOAc<br />
H<br />
AcO<br />
HB<br />
H<br />
HO<br />
OC<br />
Me<br />
O<br />
H<br />
H<br />
OC<br />
Me<br />
HO<br />
O<br />
H<br />
H<br />
CO 2Me<br />
HO<br />
H<br />
H<br />
H<br />
CO 2Me<br />
e.g. GA 35<br />
H<br />
OH<br />
OH<br />
OH<br />
H
OC<br />
Me<br />
O H<br />
H<br />
CO 2Me<br />
Br OH<br />
H<br />
OC<br />
H<br />
Me<br />
OH<br />
Br<br />
OAc<br />
Br O<br />
CO 2H<br />
19-GA-C12oxidation.cdx 2/5/04 11:32 AM<br />
O<br />
H<br />
H<br />
C12 oxidation <strong>of</strong> Gibberellin Skeleton<br />
Pb(OAc) 4, I 2<br />
OH<br />
H<br />
H<br />
Pb 4+<br />
OC<br />
Me<br />
OC<br />
Me<br />
GA 70 GA69<br />
O<br />
H<br />
O H<br />
H<br />
O<br />
CO 2Me<br />
Br OH<br />
H<br />
CO 2H<br />
H<br />
OH<br />
Zn<br />
–1e –<br />
Br<br />
OAc<br />
Br<br />
OC<br />
Zn, HOAc<br />
Me<br />
O<br />
H<br />
H<br />
O<br />
H<br />
CO 2H<br />
GA 31<br />
OH<br />
OC<br />
Me<br />
OH<br />
O<br />
H<br />
H<br />
H<br />
CO 2Me<br />
OH<br />
OH<br />
OAc
MOMO<br />
MOMO<br />
MOMO<br />
acyloin<br />
rearrangement<br />
OC<br />
Me<br />
OC<br />
O<br />
H<br />
2 steps<br />
Me<br />
OC<br />
Me<br />
H<br />
O<br />
H<br />
CO 2Me<br />
CO 2Me<br />
H<br />
CO 2Me<br />
NaOMe<br />
20-GA-C14hydroxlation.cdx 2/5/04 11:29 AM<br />
O<br />
H<br />
H<br />
C14 Hydroxylation <strong>of</strong> Gibberellin Skeleton<br />
OH<br />
O<br />
OAc<br />
OTBS<br />
OH<br />
O<br />
O<br />
O<br />
OH<br />
OH<br />
1) DMDO<br />
2) TBAF<br />
MOMO<br />
HO H<br />
O<br />
OH<br />
OC<br />
Me<br />
O<br />
H<br />
dipole<br />
minimized?<br />
OH<br />
H<br />
HO O<br />
Ab initio: ∆5.7 kcal<br />
MOMO<br />
H<br />
CO 2Me<br />
OC<br />
Me<br />
OH<br />
O<br />
OH<br />
O<br />
H<br />
NaOMe<br />
H<br />
OH<br />
CO2Me OAc<br />
Mander, Tetrahedron, 1998, 11637.<br />
O
O<br />
HO 2C<br />
9 : 1 at C4<br />
OC<br />
Me<br />
O<br />
O<br />
O<br />
H<br />
H<br />
H<br />
Me<br />
O–<br />
H<br />
CO 2Me<br />
NaOH<br />
O<br />
OC<br />
21-GA-C18hydroxlation.cdx 2/5/04 11:25 AM<br />
C18 Hydroxylation <strong>of</strong> Gibberellin Skeleton<br />
H<br />
CO 2Me<br />
O<br />
H<br />
H<br />
H<br />
H<br />
CO 2Me<br />
H<br />
AllylOH<br />
DBU<br />
HO<br />
HO<br />
OC<br />
O<br />
O<br />
H<br />
OH<br />
OC<br />
H<br />
CO 2Me<br />
O<br />
H<br />
H<br />
H<br />
RhCl(PPh 3) 3<br />
DABCO<br />
CO 2Me<br />
H<br />
2 : 3 mixture <strong>of</strong><br />
3α and 3β−OH<br />
Thomson, Mander, JCS Perkin I, 2000, 2893.
MOMO<br />
OC<br />
CH 3<br />
H<br />
OC<br />
CH 3<br />
Li/NH 3<br />
tBuOH<br />
H<br />
O OMOM<br />
H<br />
H<br />
CO 2Me<br />
CO 2Me<br />
O<br />
OMOM<br />
22-GA-C19toC20.cdx 2/5/04 11:23 AM<br />
Conversion <strong>of</strong> C 19 <strong>Gibberellins</strong> into C 20 Variants<br />
CH 3<br />
H<br />
H<br />
CO 2Me<br />
KH, DMF; O 2<br />
Li/NH 3<br />
tBuOH<br />
OMOM<br />
HO 2C<br />
HO 2C<br />
CHO<br />
H<br />
CH 3<br />
H<br />
H<br />
CH 3<br />
H<br />
CO 2Me<br />
Cu (powder)<br />
PhH, 80˚C<br />
CO 2Me<br />
C 20 gibberellins: e.g. GA 19<br />
OMOM<br />
N 2<br />
OMOM<br />
O<br />
SOCl 2;<br />
CH 2N 2<br />
H<br />
CH 3<br />
H<br />
CO 2Me<br />
OK OK O O<br />
OMOM<br />
OK<br />
O<br />
Mander, Tet. Lett. 1985, 5725.<br />
O
SnR 3<br />
PhO<br />
S<br />
RO<br />
O<br />
R 3Sn-S<br />
O<br />
O<br />
O<br />
O<br />
O<br />
CO<br />
Me<br />
CO<br />
Me<br />
OPh<br />
Me<br />
H<br />
H<br />
H<br />
H<br />
CO 2Me<br />
H<br />
CO 2Me<br />
Bu 3SnH<br />
H<br />
CO 2Me<br />
23-GA-radicalcascade.cdx 2/5/04 11:15 AM<br />
Radical Cascade: Attempted Deoxygenation at C3<br />
OAc<br />
OAc<br />
OAc<br />
Bu 3SnH<br />
R 3Sn-S<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
equatorial (α)<br />
C3 hydroxyl removed<br />
without event<br />
5-exo<br />
O<br />
O<br />
H<br />
H<br />
Me<br />
Me<br />
H<br />
H<br />
CO 2Me<br />
H<br />
H<br />
CO 2Me<br />
OAc<br />
R 3Sn-S<br />
O<br />
O<br />
OAc<br />
O<br />
O<br />
H<br />
Me<br />
H<br />
5-exo<br />
H<br />
CO 2Me<br />
OAc<br />
Barton, McCombie, JCS Perkin I, 1975, 1574.<br />
Mander,TL, 1996, 4255.<br />
*syn<strong>the</strong>tic application: Sherburn,JACS, 2003, 12108.
HO<br />
OC<br />
Me<br />
Zn<br />
O<br />
H<br />
90%<br />
F 3C<br />
H<br />
CO 2Me<br />
TsCl, pyr<br />
TsO<br />
O<br />
HO<br />
OC<br />
Me<br />
24-GA3-coreytotal1.cdx 2/5/04 12:45 PM<br />
Dismantling and Reconstituting <strong>the</strong> A-Ring<br />
O<br />
OH<br />
H<br />
methyl gibberellate<br />
HO2C CO2Me Corey-Carney Acid<br />
O<br />
H<br />
I<br />
OC<br />
Me<br />
H<br />
CO 2Me<br />
O<br />
H<br />
H<br />
CO 2Me<br />
OH<br />
NaBr,<br />
HMPA<br />
OH<br />
Br<br />
OC<br />
Me<br />
O<br />
H<br />
1) I 2, NaHCO 3<br />
2) TFAA, pyr<br />
60%<br />
H<br />
CO 2Me<br />
HO<br />
HO<br />
HO 2C<br />
CH 3<br />
OH<br />
Zn<br />
H<br />
CH 3<br />
H<br />
H<br />
CO 2Me<br />
OH<br />
1) mCPBA<br />
2) NaOH<br />
OH<br />
76%<br />
Danheiser, Strategies and Tactics in Organic Syn<strong>the</strong>sis.<br />
Ed. Lindberg 1984, 22-65.
THPO<br />
O<br />
OMe<br />
o-allyl eugenol<br />
K, TiCl 3<br />
(50%)<br />
H<br />
Corey Syn<strong>the</strong>sis <strong>of</strong> GA 3: Hydronaphthalene Approach<br />
THPO<br />
OH<br />
OH<br />
7 steps<br />
H<br />
BnO<br />
CHO<br />
"...quenching reactions involving<br />
50g <strong>of</strong> potassium can provide moments<br />
<strong>of</strong> great drama, as well as piquant stimulation<br />
for <strong>the</strong> experimentalist."<br />
25-GA3-coreytotal2.cdx 2/5/04 12:47 PM<br />
O<br />
1) Cl 3CCO) 2O,<br />
NEt 3, DMSO<br />
2) MEMCl, iPr 2NEt<br />
60%<br />
O<br />
O<br />
THPO<br />
OMe<br />
1) H 2, Rh/C<br />
2) Li, NH 3<br />
3) PDC<br />
50% <strong>from</strong> [4+2] adduct<br />
H<br />
HO<br />
THPO<br />
O<br />
H<br />
BnO<br />
OMEM<br />
PhH, 80˚C<br />
90%<br />
OMOM<br />
O<br />
HO<br />
H<br />
BnO<br />
Oxidation Products:<br />
H<br />
H<br />
COR<br />
OH<br />
O<br />
O<br />
O<br />
O<br />
1) DHP, TsOH<br />
2) NaBH 4<br />
H<br />
H<br />
OMe<br />
3) MOMCl, iPr 2NEt<br />
4) LAH<br />
5) MsCl, NEt 3 ; H 2O<br />
R=H / OH<br />
O<br />
O<br />
Corey, JACS, 1978, 8034.<br />
OH<br />
OH
Cl<br />
THPO<br />
O<br />
O<br />
Contraction <strong>of</strong> B-Ring; A-Ring Formation through Cycloaddition<br />
H<br />
H<br />
O<br />
OMEM<br />
OMEM<br />
26-GA3-coreytotal3.cdx 2/5/04 12:51 PM<br />
1) OsO 4, NMMO<br />
2) Pb(OAc) 4<br />
nBuLi;<br />
O<br />
Cl Cl<br />
72%<br />
OHC<br />
OHC<br />
H<br />
OMEM<br />
89%<br />
THPO<br />
O<br />
78%<br />
HO<br />
H<br />
OMEM<br />
1) Ph 3PCH 2<br />
HMPA, 65˚<br />
2) AcOH<br />
57%<br />
160˚C, PhH<br />
H<br />
H<br />
OMEM<br />
LiN(iPr)C6H11; O<br />
Cl<br />
H<br />
MeI<br />
55%<br />
O<br />
O<br />
70%<br />
Bn 2NH 2 + -TFA –<br />
OHC<br />
O<br />
THPO<br />
Me<br />
H<br />
O<br />
H<br />
H<br />
OMEM<br />
O<br />
OMEM<br />
Corey, JACS, 1978, 8034.
HO<br />
HO<br />
O<br />
Me<br />
I<br />
OC<br />
Me<br />
H<br />
O<br />
O<br />
H<br />
H<br />
H<br />
CO 2Me<br />
OMEM<br />
OH<br />
1) TFAA, pyr<br />
2) Zn<br />
3) PrSLi<br />
27-GA3-coreytotal4.cdx 2/5/04 12:53 PM<br />
Gibberellic Acid Endgame: Corey<br />
1) ZnBr 2<br />
2) KOH, Na 2RuO 4<br />
HO<br />
OC<br />
Me<br />
95%<br />
1) mCPBA<br />
2) NaOH<br />
3) I 2, NaHCO 3<br />
O<br />
H<br />
H<br />
CO 2H<br />
OH<br />
HO 2C<br />
HO 2C<br />
GA 3<br />
H<br />
Me<br />
H<br />
Me<br />
H<br />
CO 2H<br />
H<br />
CO 2Me<br />
OH<br />
TsCl, NEt 3;<br />
MeOH<br />
OH<br />
Corey-Carney Acid<br />
Corey, JACS, 1978, 8034.
O<br />
Alternative Route to Key Tricyclic Intermediate: The Hammer and Tongs Approach<br />
O<br />
Me<br />
O<br />
O<br />
6 steps<br />
H<br />
H<br />
48%<br />
OBn<br />
OMEM<br />
O<br />
OH Me<br />
O<br />
3 steps 7 steps<br />
OBn<br />
67% 60%<br />
NaOH,<br />
EtOH<br />
1) EtOCHO, NaH<br />
2) KOtBu, MeI<br />
88%<br />
28-GA3-coreytotalrevised1.cdx 2/5/04 12:54 PM<br />
46% 4 steps<br />
O<br />
O<br />
O<br />
OMe<br />
H<br />
H<br />
O<br />
Me<br />
O<br />
OMEM<br />
3 steps<br />
O<br />
O<br />
1) EtOCHO, NaH<br />
2) RedAl<br />
3) H +<br />
4) Ph 3PCH 2<br />
39%<br />
O<br />
O<br />
H<br />
H<br />
OH<br />
OMs<br />
O<br />
KOtBu<br />
93%<br />
O<br />
Corey, JACS, 1978, 8034.<br />
OMEM
O<br />
H<br />
1) BuLi;<br />
2) DBU<br />
Br<br />
OMEM<br />
enantioselective variant<br />
has appeared<br />
Br Br<br />
Br Br CHO<br />
Cope Rearrangement for B/C Ring Junction<br />
9 steps saved over original<br />
syn<strong>the</strong>sis<br />
29-GA3-coreytotalrevised2.cdx 2/5/04 12:58 PM<br />
1) nBu 2CuLi<br />
2) MEMCl, iPr 2NEt<br />
65%<br />
R<br />
O<br />
H<br />
O<br />
N B<br />
Ts<br />
nBu<br />
10% mol<br />
99%ee<br />
81%<br />
R = 3-indole<br />
MeO2C Me<br />
+ C2 isomer<br />
2: 1<br />
1) BF3•OEt2 87%<br />
2) TMSOTf, NEt3 53%<br />
O<br />
Br<br />
H<br />
Br O<br />
CHO<br />
Br<br />
O<br />
1) 9BBN;<br />
NaOH, H 2O 2<br />
2) PDC<br />
5 steps<br />
76%<br />
Br<br />
[3,3]<br />
Br<br />
CO 2Me<br />
OTMS<br />
160˚C<br />
DMSO, H2O NaCl<br />
71%<br />
H<br />
Br O<br />
CO 2Me<br />
OTMS<br />
Corey, JACS, 1982, 6129.<br />
Corey, JACS, 1994, 3611.
MeO<br />
HO 2C<br />
MeO<br />
TFA<br />
MeO<br />
CO 2Me<br />
35%<br />
CO 2Me<br />
OMe<br />
30-GA3-mandertotal1.cdx 2/5/04 1:00 PM<br />
MeO<br />
Mander: Fluorene Approach<br />
1) Li, NH 3<br />
2)<br />
CO 2Me<br />
OCOCH 2Cl<br />
O<br />
O<br />
N 2<br />
OCOCH 2Cl<br />
I<br />
MeO<br />
MeO<br />
OMe<br />
CO2H CO<br />
88% 2Me<br />
36%<br />
1) HCN; NaOH<br />
2) (ClCH 2CO) 2O<br />
3) (ClCO) 2; CH 2N 2<br />
64%<br />
1) Na 2CO 3, MeOH<br />
2) H + , (HOCH 2) 2<br />
3) MOMCl, iPr 2NEt<br />
4) tBu(chx)NLi; CO 2<br />
5) H 2, Pd/C<br />
68%<br />
MeO<br />
MeO<br />
PPA<br />
CO 2Me<br />
CO 2Me<br />
H<br />
CO 2H<br />
OMOM<br />
O<br />
O<br />
O<br />
Mander, JACS. 1980, 6626.
MeO<br />
PhOCO<br />
MeO<br />
Mander: A-Ring Assembly through Birch Reduction/Alkylation<br />
CO 2Me<br />
OC<br />
Br<br />
Me<br />
CO 2H<br />
O<br />
H<br />
CO 2H<br />
H<br />
CO 2Et<br />
H<br />
CO 2Me<br />
31-GA3-mandertotal2.cdx 2/5/04 1:03 PM<br />
OMOM<br />
O<br />
O<br />
O<br />
O<br />
OMOM<br />
O<br />
O<br />
1) KOtBu, K, NH 3; MeI<br />
2) CH 3CHN 2<br />
66%<br />
KHCO 3, KBr 3<br />
86%<br />
Na, NH 3; MeI<br />
C7 ester controls alkylation<br />
MeO<br />
MeO<br />
MeO 2C<br />
MeO 2C<br />
PhOCO<br />
HO 2C<br />
Me<br />
Me<br />
H<br />
Me<br />
CO 2Me<br />
H<br />
CO 2Et<br />
H<br />
CO 2Et<br />
O<br />
O<br />
OMOM<br />
O<br />
O<br />
OMOM<br />
O<br />
O<br />
4 steps<br />
Mander, JACS. 1980, 6626.<br />
Baker, Chem. Com. 1972, 951.
PhOCO<br />
OC<br />
1) DBU<br />
2) H 2O, H +<br />
3) TMSCl<br />
90%<br />
O<br />
Br<br />
Me<br />
PhOCO<br />
H<br />
CO 2Et<br />
PhOCO<br />
OC<br />
Br<br />
H<br />
Me<br />
31B-GA3-mandertotal3.cdx 2/6/04 10:12 AM<br />
O<br />
OMOM<br />
O<br />
O<br />
OC<br />
H<br />
Me<br />
H<br />
O<br />
CO 2Me<br />
Mander: Gibberellic Acid<br />
5 steps<br />
H<br />
CO 2Me<br />
OTMS<br />
O<br />
OH<br />
O<br />
O<br />
OC<br />
O<br />
H<br />
Me<br />
H<br />
CO 2Me<br />
NBS, hν<br />
95%<br />
1) Ph 3PCH 2,<br />
ClCH 2CH 2OTMS<br />
2) K 2CO 3, MeOH<br />
3) nPrSLi, HMPA<br />
75%<br />
OH<br />
O<br />
O<br />
O<br />
PhHC<br />
O<br />
HO<br />
1) OsO 4, NMMO<br />
2) PhCHO, H +<br />
OC<br />
OC<br />
Me<br />
H<br />
Me<br />
O<br />
H<br />
O<br />
H<br />
H<br />
CO 2Me<br />
CO 2H<br />
OH<br />
OH<br />
O<br />
O<br />
GA 3<br />
Mander, JACS. 1980, 6626.
O<br />
O<br />
Me<br />
89%<br />
OC<br />
Me<br />
O<br />
OMe<br />
O<br />
H<br />
OCOCCl 3<br />
O<br />
H<br />
N 2<br />
CO 2Me<br />
H<br />
CO 2Me<br />
OH<br />
O<br />
O<br />
TFA<br />
1) KH, Et 3NH-OAc<br />
2) (sia) 2BH; NaOOH<br />
3) PDC<br />
OH<br />
O<br />
32-GA3-mandertotal4.cdx 2/5/04 1:09 PM<br />
O<br />
Mander: A-ring Aldol Approach (GA 1)<br />
OCOCCl 3<br />
O<br />
O<br />
99% O<br />
49% O<br />
80%<br />
1) (H 2C=CHCH 2) 3Al<br />
2) (EtCO) 2O, DMAP<br />
78%<br />
K 2CO 3,<br />
MeOH<br />
1:1 at C3<br />
60%<br />
HO<br />
OC<br />
O<br />
Me<br />
O<br />
H<br />
4 steps<br />
H<br />
CO 2Me<br />
H<br />
CO 2Me<br />
N 2<br />
OH<br />
O<br />
O<br />
OH<br />
O<br />
O<br />
HO<br />
OH<br />
O<br />
1) (sia) 2BH;<br />
NaOOH<br />
2) PDC<br />
3) LDA; Ph 2Se 2<br />
4 steps<br />
50%<br />
OC<br />
Me<br />
O<br />
H<br />
hv,MeOH<br />
CO 2Me<br />
GA 1<br />
H<br />
CO 2Me<br />
OH<br />
O<br />
O<br />
OH<br />
Mander, JACS. 1980, 6626.
Me<br />
SEMO<br />
MOMOH 2C<br />
CN<br />
CO 2Me<br />
H<br />
Me<br />
O 3, MeOH<br />
86%<br />
H<br />
CN<br />
Yamada: Intermolecular [4+2] Ring A Construction<br />
H<br />
OMe<br />
CH 2OMOM<br />
SEMO<br />
MOMOH 2C<br />
2) NaOH<br />
3) Ac 2O<br />
57%<br />
33-GA3-yamadatotal.cdx 2/5/04 2:48 PM<br />
O<br />
H<br />
H<br />
Me<br />
Me<br />
allene,<br />
hν<br />
69%<br />
H<br />
O<br />
O<br />
H<br />
SEMO<br />
MOMOH 2C<br />
O<br />
CO 2Me<br />
CH 2OMOM<br />
O<br />
H<br />
H<br />
Me<br />
OMe<br />
H<br />
1) K, NH 3<br />
2) Swern<br />
CH 2OMOM<br />
1) AlCl 3<br />
2) mCPBA<br />
O<br />
49%<br />
Nakanishi, Chem. Com, 1969, 528.<br />
3) MOMCl, iPr 2NEt<br />
4) Ph 3PCH 2<br />
53%<br />
TMSO<br />
SEMO<br />
MOMOH 2C<br />
OC<br />
1) Na, NH 3<br />
2) AcOH, H 2O<br />
O<br />
H<br />
Me<br />
3) K 2CO 3, MeOH<br />
80%<br />
O<br />
SEMO<br />
MOMOH 2C<br />
H<br />
H<br />
Me<br />
H<br />
H<br />
H<br />
Me<br />
CH 2OMOM<br />
OMe<br />
10 steps<br />
CH 2OMOM<br />
OMOM<br />
OMe<br />
Yamada, TL, 1989, 971.
SEMO<br />
MOMOH 2C<br />
91%<br />
HO 2C<br />
OC<br />
H<br />
H<br />
Me<br />
H<br />
Me<br />
O<br />
H<br />
Me<br />
H<br />
CH 2OMOM<br />
8 steps<br />
H<br />
CO 2H<br />
H<br />
CO 2H<br />
34-GA3-yamadatotal2.cdx 2/5/04 2:50 PM<br />
Yamada: Syn<strong>the</strong>sis <strong>of</strong> Gibberellic Acid<br />
1) I 2, NaHCO 3<br />
2) DBU<br />
OMOM<br />
OMOM<br />
OMOM<br />
1) MOMCl, iPr 2NEt<br />
2) LDA<br />
99%<br />
HO<br />
HO 2C<br />
OC<br />
O<br />
H<br />
Me<br />
H<br />
Me<br />
H<br />
CO 2H<br />
H<br />
CO 2MOM<br />
OH<br />
GA 3<br />
6 steps (Corey et al)<br />
MOM-protected<br />
Corey-Carney Acid<br />
OMOM<br />
30% <strong>from</strong> tri-MOM-e<strong>the</strong>r<br />
Yamada, TL, 1989, 971.
CO 2Me<br />
O<br />
OMe<br />
H<br />
EtO2C MeO<br />
52%<br />
OC<br />
O<br />
H<br />
MeO<br />
H<br />
H<br />
O<br />
<strong>the</strong>n<br />
LiN(chx)iPr;<br />
MeI<br />
Br<br />
MgI<br />
OMEM<br />
OMEM<br />
36-GA3-DeClerqtotal.cdx 2/5/04 4:29 PM<br />
Syn<strong>the</strong>sis <strong>of</strong> GA 5 via Furan [4+2]: DeClercq<br />
Br<br />
5 steps<br />
81%<br />
1) PPTS<br />
2) NaClO 2<br />
50%<br />
46%<br />
O Br<br />
MeO 2C<br />
O<br />
CO 2Et<br />
OC<br />
Me<br />
O<br />
H<br />
H<br />
OH<br />
H<br />
CO 2H<br />
O<br />
kinetic<br />
product<br />
OH<br />
OH<br />
GA 5<br />
Bu 2CuLi<br />
65%<br />
O<br />
β-cyclodextrin<br />
H 2O, 65˚C<br />
96%<br />
>95:5<br />
MeO 2C<br />
EtO 2C<br />
O<br />
O<br />
CO 2Et<br />
OH<br />
3 steps<br />
50%<br />
H<br />
OH<br />
OH<br />
PhH<br />
80˚C<br />
OH<br />
OH<br />
<strong>the</strong>rmodynamic<br />
product<br />
DeClercq, Tet. Lett. 1986, 1731.
MeO 2C<br />
Me<br />
H<br />
Me<br />
Me<br />
Me<br />
(–)-methyl dehydroabietate<br />
1) H 2, RuO 2<br />
2) H 2CrO 4<br />
3) Ph 3PCH 2<br />
4) BH 3/H 2O 2<br />
H<br />
MeO2C Me<br />
epimerization at C6<br />
Me<br />
H<br />
MeO2C Me<br />
CO 2Me<br />
Me<br />
OH<br />
37-GA12-Tahara-total.cdx 2/5/04 3:00 PM<br />
H<br />
H<br />
GA 12 Syn<strong>the</strong>sis <strong>from</strong> Dehydroabiatate: Tahara<br />
AlCl 3<br />
39%<br />
CO 2Me<br />
1) H 2CrO 4<br />
2) SOCl 2;<br />
CH 2N 2<br />
MeO 2C<br />
Me<br />
Me<br />
H<br />
Wenkert, JACS, 1958, 211.<br />
OH 1) H2, Pd/C<br />
2) AcCl, AlCl3 3) mCPBA;<br />
NaOH<br />
Me<br />
H<br />
MeO2C Me<br />
H<br />
Me<br />
MeO 2C Me<br />
H<br />
CO 2Me<br />
CrO 3, HOAc<br />
N 2<br />
O<br />
CO 2Me<br />
CuSO 4,<br />
hν<br />
1) CH 2N 2<br />
2) H 2SO 4<br />
Me<br />
H<br />
MeO2C Me O<br />
H<br />
MeO2C Me<br />
O<br />
Me<br />
H<br />
MeO2C Me<br />
Me H<br />
H<br />
CO 2Me<br />
GA 12<br />
KOH<br />
OH<br />
CO2H O<br />
Tahara, JCS Perkin I, 1972, 320.<br />
Tahara, TL, 1976, 1515.<br />
4 steps
MeO 2C<br />
Me<br />
H<br />
Me<br />
H<br />
(±)- <strong>from</strong> syn<strong>the</strong>sis <strong>of</strong><br />
steviol Tet ,1966, 879.<br />
Me<br />
O<br />
Me<br />
H<br />
H<br />
O<br />
MeO 2C<br />
H<br />
O<br />
O<br />
Cross, Hanson, JCS, 1963, 2944.<br />
1) NaBH 4<br />
2) TsCl, pyr<br />
38-GA12-Mori-total.cdx 2/5/04 3:03 PM<br />
4 steps<br />
Me<br />
O<br />
GA 12 Formal Syn<strong>the</strong>sis: Mori<br />
Me<br />
H<br />
Me<br />
1) Br 2, HOAc<br />
2) LiCl, DMF<br />
3) Ph 3PCH 2<br />
Me<br />
H<br />
H<br />
O<br />
H<br />
H<br />
MeO 2C<br />
H<br />
OTs<br />
O<br />
O<br />
Me<br />
H<br />
Me<br />
1) Ph 3PCHOAr<br />
2) H 3O +<br />
3) Ph3PCH2 10-30%<br />
H<br />
O<br />
H<br />
O<br />
1) KOH, tBuOH<br />
2) H 2CrO 4<br />
MeO 2C<br />
Me<br />
H<br />
Me<br />
1) NaH<br />
2) H 3O +<br />
3) H 2CrO 4<br />
10%<br />
H<br />
H<br />
HO2C Me<br />
H<br />
MeO 2C<br />
O<br />
CO 2H<br />
Me<br />
H<br />
Me<br />
H<br />
Me H<br />
H<br />
1) NBS, H 2O<br />
2) DHP, H +<br />
H<br />
O<br />
GA 12<br />
H<br />
OTHP<br />
Br<br />
Mori, Tet, 1976, 1497.
H<br />
Me<br />
Me<br />
O<br />
O<br />
10 steps<br />
37%<br />
Me<br />
H<br />
H<br />
O<br />
Me<br />
H<br />
MeO2C Me<br />
H<br />
H<br />
O<br />
1) Bu 3SnH,<br />
AIBN<br />
2) SiO 2<br />
H<br />
H<br />
Me<br />
Me<br />
O<br />
H<br />
H<br />
HO2C Me<br />
Me H<br />
H<br />
CO 2H<br />
H<br />
Me H<br />
OAc<br />
2) TBAF<br />
Me<br />
OAc<br />
OTES<br />
3:1 at C7<br />
OTES<br />
72% (4 steps)<br />
2) Ac2O Cross, Hanson, JCS, 1963, 2944.<br />
39-GA12-Ihara-formal.cdx 2/5/04 3:04 PM<br />
GA 12 Formal Syn<strong>the</strong>sis: Ihara<br />
1) 200˚C<br />
O<br />
GA 12<br />
SnR 3<br />
Me<br />
1) s-BuLi<br />
O<br />
H<br />
Me<br />
Me H<br />
Me<br />
O<br />
O<br />
A : B<br />
93% (1:18 mix)<br />
O<br />
H<br />
AcO<br />
H<br />
OTES<br />
Me<br />
O<br />
H<br />
5 steps<br />
88%<br />
Ihara, JACS, 2001, 1856.
O<br />
O<br />
H<br />
O<br />
O<br />
Three Routes to Bicycle:<br />
O<br />
O<br />
O<br />
O OEt MeO2C OEt<br />
OH<br />
40-GA3-Stork-CDring.cdx 2/5/04 3:05 PM<br />
O<br />
Stork D-ring Approach: Reductive Cyclization<br />
1) H 3O +<br />
2) NaBH 4<br />
3) (HOCH 2) 2 H +<br />
4) PDC<br />
O<br />
O<br />
O<br />
Br NC<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
H<br />
O<br />
O<br />
O<br />
H<br />
O<br />
MeO 2C<br />
O<br />
O<br />
CN<br />
1) K, NH 3,<br />
(NH 4) 2SO 4<br />
2) HOAc, H 2O<br />
O<br />
H<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
H<br />
H<br />
OH<br />
O<br />
O<br />
Stork, JACS, 1979, 7107.<br />
Stork, JACS, 1965, 1148.
MeO<br />
TBSO<br />
6 steps<br />
52%<br />
TBSO<br />
CO 2Me<br />
OC<br />
O<br />
H<br />
OH<br />
H<br />
OH<br />
I<br />
O 2N<br />
O O<br />
O<br />
CO 2Me<br />
N-tBu<br />
= L<br />
NiBr<br />
2 2<br />
41-anteridiogens3-Corey.cdx 2/5/04 3:31 PM<br />
Total Syn<strong>the</strong>sis <strong>of</strong> An<strong>the</strong>ridic Acid: Corey<br />
H<br />
TBSO<br />
TBSO<br />
H<br />
EtAlCl2 51% overall<br />
O O<br />
53%<br />
O O<br />
80%<br />
O 2N<br />
7 steps<br />
76%<br />
TBSO<br />
H<br />
1) TMSCl, LDA<br />
TBSO<br />
2) Eschemoser's<br />
salt, MeI, iPr2NEt 60%<br />
OC<br />
OH<br />
H<br />
N 2<br />
O O<br />
O<br />
H<br />
O<br />
1) Cu(II)L 2<br />
2) Br 2; DBU<br />
CO 2Me<br />
1) MeCO 3H<br />
2) LiNEt 2<br />
H<br />
57%<br />
4 steps<br />
90%<br />
TBSO<br />
HO<br />
OC<br />
O<br />
H<br />
H<br />
H<br />
O O<br />
OH<br />
CO 2H<br />
an<strong>the</strong>ridic acid<br />
original structure proposed as 3β−OH<br />
Corey, Myers, JACS, 1985, 5574.<br />
H
AcO<br />
HO2C Proposed Biomimmetic Syn<strong>the</strong>sis <strong>of</strong> An<strong>the</strong>ridic Acid Investigated<br />
H<br />
CO 2Me<br />
A or B<br />
AcO<br />
MeO2C A) Im 2CO, H 2O 2 intramolecular delivery<br />
B) mCPBA (k rel < 10 -2 ) intermolecular<br />
42-anteridiogens2.cdx 2/5/04 3:33 PM<br />
C9,10-epoxygibberellin<br />
H<br />
O<br />
CO 2Me<br />
Epoxide initiated<br />
1,2 bond migration<br />
Desired Bond Migration<br />
could not beEffected<br />
HO<br />
AcO<br />
MeO2C OC<br />
O<br />
H<br />
OH<br />
H<br />
OH<br />
CO 2H<br />
an<strong>the</strong>ridic acid<br />
original structure<br />
proposed as<br />
3β−OH<br />
CO 2H<br />
H<br />
Mander, JACS, 1987, 6839.<br />
H
HO<br />
MOMO<br />
MOMO<br />
OC<br />
O I<br />
O<br />
CO<br />
CO<br />
O<br />
H<br />
H<br />
KH<br />
H<br />
CO 2H<br />
43-anteridiogens.cdx 2/5/04 3:35 PM<br />
H<br />
CO 2Me<br />
CO 2Me<br />
Conversion <strong>of</strong> GA 7 into An<strong>the</strong>ridic Acid<br />
H<br />
GA 7<br />
H<br />
H<br />
O<br />
O<br />
4 steps<br />
HO<br />
OC<br />
an<strong>the</strong>ridic acid<br />
original structure<br />
proposed as<br />
3β−OH<br />
MOMO<br />
MOMO<br />
OC<br />
OC<br />
O<br />
H<br />
O<br />
H<br />
O<br />
H<br />
OH<br />
CO 2H<br />
CO 2Me<br />
CO 2Me<br />
H<br />
O<br />
H<br />
1) DBU<br />
2) H2, Rh<br />
O<br />
3) Ph3PCH2 H<br />
1) LiN(chx)iPr;<br />
Et 3NHCl<br />
2) SeO 2, tBuOOH<br />
3) Me 2BBr<br />
4) LiOH<br />
Mander, JACS, 1987, 6839.
AcO<br />
75%<br />
OC<br />
O<br />
H<br />
an<strong>the</strong>ridic acid<br />
OMe<br />
COCHN 2<br />
CO 2Me<br />
Cu(acac) 2<br />
1) PhI(OAc) 2, I 2<br />
2) Hg(OAc) 2 HOAc<br />
Formal<br />
syn<strong>the</strong>sis<br />
44-an<strong>the</strong>ridiogens4-Mander.cdx 2/5/04 3:36 PM<br />
O<br />
H<br />
Syn<strong>the</strong>sis <strong>of</strong> An<strong>the</strong>ridiogens: Mander<br />
HO 2C<br />
71%<br />
OC<br />
OC<br />
O<br />
H<br />
O<br />
OMe<br />
H<br />
CO 2Me<br />
O<br />
1) PhI(OAc) 2, I 2<br />
2) HSnBu 3<br />
3) Zn, CH 2Br 2<br />
TiCl 4<br />
O<br />
H<br />
CO 2H<br />
H<br />
O<br />
H<br />
O<br />
O<br />
Me<br />
18h, rt<br />
added in situ<br />
GA 103<br />
75%<br />
1) hν, MeOH<br />
2) PDC<br />
3) H 2, Pd<br />
70%<br />
O<br />
O<br />
BnO 2C<br />
OH<br />
O<br />
OC<br />
H<br />
O<br />
H<br />
5 steps<br />
31%<br />
OC<br />
CO 2H<br />
O<br />
H<br />
N 2<br />
H<br />
O<br />
OMe<br />
HO<br />
H<br />
O<br />
GA 104<br />
H<br />
Mander, JACS, 1997, 3828.
H<br />
H<br />
O<br />
H<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
NaOMe<br />
H<br />
O<br />
H<br />
H<br />
O<br />
O<br />
O<br />
OH<br />
Me<br />
OH<br />
OH<br />
tBuMgCl<br />
H<br />
OH<br />
SMe<br />
14 steps<br />
MeO2C O SMe<br />
O<br />
MeO2C O O<br />
CO2Me House, JOC, 1973, 1398.<br />
O<br />
Zn,<br />
HOAc<br />
H<br />
OH<br />
H Br<br />
CO2Me Ziegler, JOC, 1971, 3707. (model system)<br />
MeO 2C<br />
CO 2Me<br />
45-cd-ring stragedy.cdx 2/5/04 3:43 PM<br />
Aldol C/D Ring Strategies Not Discussed<br />
Hg(II)O<br />
H 2SO 4<br />
HCl,<br />
acetone<br />
MeO 2C<br />
Ireland, JOC, 1966, 2530. (toward kaurenes)<br />
Takano, Ogasawara, Chem. Com., 1981, 635.<br />
Takano, Ogasawara, Chem. Com., 1981, 637.<br />
H<br />
OH
H<br />
MeO2C H<br />
O<br />
CO2Me 1) NaOMe<br />
CO2Me2) HCl<br />
O<br />
CO 2H<br />
CO 2H<br />
46-cd-ring stragedy2.cdx 2/5/04 3:44 PM<br />
Acylation C/D Ring Strategies Not Discussed<br />
BF 3<br />
AcOH<br />
PPA<br />
H<br />
H<br />
CO 2Me<br />
H<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Baker, Chem. Com., 1971, 180.<br />
Lowenthal, JCS Perkin I, 1976, 944.<br />
Jammaer, Tet., 1975, 2293.
O<br />
H<br />
H<br />
MeO<br />
O<br />
O<br />
O<br />
OMs<br />
OTs<br />
CHO<br />
SPh<br />
DBU<br />
O<br />
H<br />
N<br />
H<br />
O<br />
MeS O<br />
O<br />
MeS O<br />
47-cd-ring stragedy3.cdx 2/5/04 3:46 PM<br />
Alkylation C/D Ring Strategies Not Discussed<br />
TFAA<br />
SnCl 4<br />
H<br />
O<br />
TFAA<br />
SPh<br />
O<br />
O<br />
O<br />
MeO<br />
CHO<br />
1) PhSCH 2Li<br />
2) TsOH<br />
6 steps<br />
SMe<br />
O<br />
H<br />
O<br />
H<br />
SMe<br />
CO 2Me<br />
O<br />
O<br />
SPh<br />
O<br />
GA 15<br />
Nagata, JACS, 1972, 4654.<br />
Trost, JOC, 1978, 1031.<br />
Mander, Syn<strong>the</strong>sis, 1981, 620.<br />
Barco, Tet., 1989, 3935.
H<br />
H<br />
MeO<br />
O<br />
Me<br />
Cl<br />
H<br />
OH<br />
O<br />
Me<br />
OH<br />
OMs<br />
CO 2H<br />
Ar<br />
CN<br />
H<br />
PCl 5<br />
H 3O +<br />
Cl<br />
O<br />
HO 2C<br />
O<br />
CN<br />
H O Zn, HOAc H<br />
48-cd-ring stragedy4.cdx 2/5/04 3:57 PM<br />
Rearrangement C/D Ring Strategies Not Discussed<br />
H 2O, acetone,<br />
2,6-Lutidine<br />
H<br />
poor conversion<br />
Ar<br />
H<br />
BF 3•OEt 2<br />
OH<br />
Me<br />
OH<br />
OH<br />
H<br />
H<br />
Cl<br />
OH CN<br />
Cross, MacMillan, JCS, 1958, 2520.<br />
OH<br />
O<br />
OH<br />
Ziegler, Tet., 1977, 373.<br />
Monti, JOC, 1978, 4062.<br />
Yamada, Syn<strong>the</strong>sis, 1977, 581.<br />
Mori, Tet., 1972, 3217.
49-cd-ring summary.cdx 2/5/04 3:58 PM<br />
A Summary <strong>of</strong> General C/D Ring Strategies<br />
H H<br />
I) Reductive Ring Closure<br />
II) Alkylation / Acylation<br />
III) Aldol<br />
IV) Carbenoid<br />
OH<br />
V) Rearrangement / Fragmentation<br />
H<br />
OH<br />
O<br />
"The problem <strong>of</strong> <strong>the</strong> syn<strong>the</strong>sis<br />
<strong>of</strong> gibberellic acid has provided <strong>the</strong> impetus for<br />
<strong>the</strong> development <strong>of</strong> many<br />
new syn<strong>the</strong>tic methods . . . " Corey