Download PDF - SynCardia Systems, Inc.
Download PDF - SynCardia Systems, Inc.
Download PDF - SynCardia Systems, Inc.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2<br />
Both failing<br />
ventricles<br />
are removed.<br />
The four<br />
native valves<br />
are removed.<br />
The atria, aorta<br />
and pulmonary<br />
artery remain<br />
intact.<br />
Quick connects are<br />
sewn into the atria,<br />
aorta and pulmonary<br />
artery.<br />
The Total Artificial<br />
Heart (TAH) is implanted<br />
and attached via four<br />
quick connects.<br />
When a donor heart<br />
becomes available,<br />
the TAH and quick<br />
connects are<br />
removed.<br />
The donor<br />
heart is<br />
transplanted.<br />
For patients dying from end-stage biventricular<br />
heart failure, there are only two options:<br />
1. An immediate donor heart transplant, availability uncertain.<br />
2. The <strong>SynCardia</strong> temporary Total Artificial Heart as a bridge to transplant,<br />
Donor Hearts: Only 3,500 Available Worldwide Annually<br />
# of Donor Heart Transplants<br />
immediately available at <strong>SynCardia</strong> Certified Centers.<br />
2500 –<br />
2000 –<br />
1500 –<br />
1000 –<br />
500 –<br />
US Donor Heart Transplants<br />
0 – 90 95 00 05 10<br />
Year<br />
Despite growing demand worldwide, only 3,500 donor hearts become available annually.<br />
It is estimated that each year, up to 100,000 people in the U.S. alone could benefit<br />
from mechanical circulatory support devices such as the <strong>SynCardia</strong> Total Artificial Heart.<br />
<strong>SynCardia</strong> Total Artificial Heart<br />
Originally used as a permanent replacement heart, the Total Artificial Heart is currently<br />
approved as a bridge to transplant for transplant-eligible patients at risk of imminent<br />
death from biventricular failure. It is the world’s first and only FDA, Health Canada and<br />
CE approved Total Artificial Heart.<br />
Bridge to Second Transplant<br />
The <strong>SynCardia</strong> Total Artificial<br />
Heart is the only device that<br />
allows surgeons to remove the<br />
failing transplanted heart and<br />
stop immunosuppressant<br />
medication until a second<br />
matching donor heart can be<br />
transplanted.<br />
“I have witnessed several patients who needed a second transplantation,<br />
but died before a donor heart became available. Those patients would<br />
have been excellent candidates for an immediately-available heart.<br />
The Total Artificial Heart is the only device that allows us to remove the<br />
failing donor heart completely and bridge patients to a second transplant<br />
without immunosuppressive therapy.”<br />
David Luís Simón Morales, MD<br />
Executive Co-Director, The Heart Institute<br />
Professor and Endowed Chair of Pediatric Cardiothoracic Surgery<br />
Chief, Pediatric Cardiothoracic Surgery<br />
Cincinnati Children’s Hospital Medical Center<br />
The University of Cincinnati College of Medicine<br />
Survival (%)<br />
100<br />
80<br />
60<br />
40<br />
20<br />
Survival Curve After Heart Transplantation<br />
N = 66,751<br />
# of Donor Heart Transplants<br />
Europe Donor Heart Transplants<br />
2500 –<br />
2000 –<br />
1500 –<br />
1000 –<br />
500 –<br />
0 – 01 02 03 04 05 06 07 08<br />
Year<br />
0<br />
Years 0 4 8 12 16 21<br />
Source: Journal of the American College of Surgery 2006; 203(2): 226-39.
Human Heart<br />
Similar to a heart transplant, the <strong>SynCardia</strong> Total Artificial Heart<br />
is the only device that:<br />
Eliminates the symptoms & source of end-stage biventricular heart failure<br />
Replaces both failing ventricles<br />
Replaces all four heart valves<br />
Eliminates native heart complications<br />
No ventricle dysfunction/failure<br />
No diseased heart valves<br />
No arrhythmias or need for pacemaker/defibrillator<br />
Provides immediate, safe blood flow up to 9.5 L/min through each ventricle<br />
Immediately restores normal Cardiac Index (CI)<br />
Immediately restores normal Central Venous Pressure (CVP)<br />
Overcomes high Pulmonary Arterial Pressure (PAP)<br />
Restores normal hemodynamics and organ perfusion<br />
Unlike a donor heart, the <strong>SynCardia</strong> Total Artificial Heart<br />
Is immediately available at <strong>SynCardia</strong> Certified Centers<br />
Does not require expensive, immunosuppressant medication, which can cause subsequent complications<br />
Mr. Okeke received his first heart transplant at age 30 after a blood clot damaged<br />
his coronary artery. Thirteen years later, his body began rejecting his donor heart and he was<br />
implanted with the Total Artificial Heart. Since receiving his second heart transplant in January 2011,<br />
Mr. Okeke has been enjoying life at home with his wife Natalie and their three young children.<br />
Charles Okeke UNITED STATES<br />
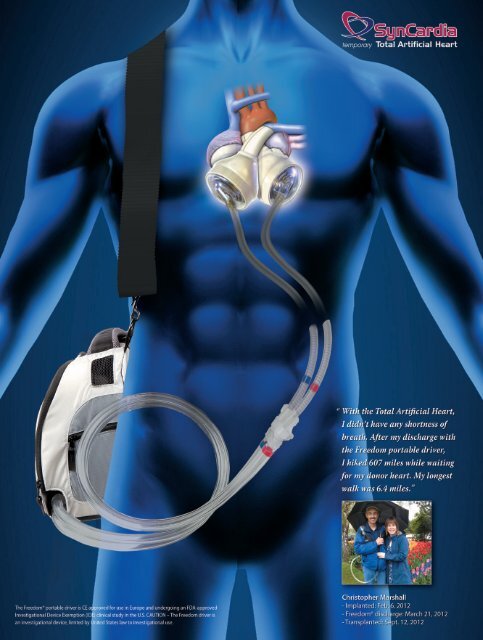
<strong>SynCardia</strong> temporary<br />
Total Artificial Heart<br />
Implanted: Sept. 3, 2008<br />
Freedom® discharge: May 3, 2010<br />
Transplanted: Jan. 15, 2011<br />
CAUTION - The Freedom® portable driver is an investigational device, limited by United States law to investigational use.<br />
3
Patients Recover Rapidly with the <strong>SynCardia</strong> Total Artificial Heart<br />
Safe Blood Flow Up to 9.5 Liters per Minute<br />
The Total Artificial Heart is the only device that provides immediate,<br />
laminar blood flow of up to 9.5 liters per minute through each ventricle.<br />
Implanting the Total Artificial Heart helps<br />
make patients healthier transplant candidates:<br />
n Immediately increases Cardiac Index (CI)<br />
n Immediately restores normal Central Venous Pressure (CVP)<br />
n Resuscitates kidneys, liver, GI tract, brain, other vital organs<br />
n Overcomes high Pulmonary Arterial Pressure (PAP)<br />
n Restores normal hemodynamics and organ perfusion<br />
Data from the 10-year pivotal clinical study of the Total Artificial Heart, published in the New England Journal of Medicine<br />
(N Engl J Med 2004; 351:859-867)<br />
Immediate <strong>Inc</strong>rease in Cardiac Index Organ Recovery at Two Weeks<br />
Prior to implant, patients in need of the Total Artificial Heart had a<br />
baseline cardiac index of 1.9 L/min/m 2 .<br />
Immediately following implant, the patient’s cardiac index<br />
increased to an average of 2.9 L/min/m 2 .<br />
Liver function had returned to normal at two weeks<br />
Kidney function had improved significantly, trending to normal<br />
Physical Recovery Bridging the Sickest Patients to Transplant<br />
Post-Operative Day 5: Approximately 65% of the<br />
core Total Artificial Heart patients were out of bed.<br />
According to March 2011<br />
INTERMACS data,<br />
72% of Total Artificial<br />
Heart patients were<br />
transplanted at 6 months<br />
and 75% at 12 months.<br />
Post-Operative Day 14: 60% of core Total Artificial<br />
Heart patients were walking more than 100 feet.<br />
Previous reports have shown that<br />
almost all Total Artificial Heart patients<br />
were classified in the two sickest INTERMACS<br />
categories prior to implant, yet the <strong>SynCardia</strong><br />
Total Artificial Heart had the highest bridge to<br />
transplant rate of all the devices tracked in INTERMACS.<br />
French Total Artificial Heart<br />
patient Mr. Lediouron<br />
pictured at home with the<br />
Freedom® portable driver in<br />
Sept. 2010 with his wife<br />
Karene and their two<br />
daughters, Wendy<br />
and Sidney.<br />
In comparison, approximately 25% of patients with other<br />
mechanical circulatory support devices were classified in less<br />
sick categories prior to implant.<br />
Christian Lediouron<br />
FRANCE<br />
Implanted: May 17, 2010<br />
Freedom® discharge: June 30, 2010<br />
Transplanted: March 3, 2011<br />
Highest bridge to transplant rate: Survival to transplantation was<br />
achieved in 79% of patients who received the <strong>SynCardia</strong> Total Artificial<br />
Heart according to protocol, as compared with 46% of the controls who<br />
did not receive the device. This is the highest bridge to transplant rate of<br />
any approved heart device.<br />
Overall survival: The one-year survival rate among patients who received<br />
the Total Artificial Heart was 70%, as compared with 31% among the<br />
controls. One-year and five-year survival rates after transplantation<br />
among patients who had received the Total Artificial Heart were<br />
86% and 64%.<br />
4 5
The <strong>SynCardia</strong> Total Artificial Heart powered<br />
by the Freedom® portable driver<br />
Left ventricular assist device (LVAD)<br />
On July 22, 2011, Shawn Galloway became the first of four patients<br />
to receive the Total Artificial Heart at Texas Heart Institute during a 12-day period.<br />
A month after her surgery, she was discharged home to wait for a matching donor heart with<br />
her husband Joel and their daughter Hannah. She received her heart transplant on Sept. 20, 2011.<br />
Shawn Galloway UNITED STATES<br />
Implanted: July 22, 2011<br />
Freedom® discharge: Aug. 23, 2011<br />
Transplanted: Sept. 20, 2011<br />
CAUTION - The Freedom® portable driver is an investigational device, limited by United States law to investigational use.<br />
6<br />
Additional Benefits of the<br />
<strong>SynCardia</strong> Total Artificial Heart<br />
No right ventricular dysfunction/failure<br />
No acquired von Willebrand syndrome (AVWS) reported<br />
Right Ventricular Dysfunction/Failure after LVAD Implant<br />
HeartWare HVAD - ADVANCE Clinical Study Results<br />
22%<br />
of patients developed right heart failure requiring either a<br />
Right Ventricular Assist Device (RVAD) or inotropic support*<br />
HeartMate II LVAD - Pre-Market Approval Results<br />
19%<br />
of patients developed right heart failure during the<br />
pre-market approval of the HeartMate II**<br />
*Source: Evaluation of the HeartWare HVAD Left Ventricular Assist System for the Treatment of<br />
Advanced Heart Failure: Results of the ADVANCE Bridge to Transplant Trial; Keith Aaronson,<br />
Mark Slaughter, Edwin McGee, et al. for the HeartWare ADVANCE Investigators; American Heart<br />
Association Scientific Sessions November 2010<br />
**Source: HeartMate II FDA Summary of Safety and Effectiveness<br />
http://www.accessdata.fda.gov/cdrh_docs/pdf6/P060040b.pdf<br />
“Acquired von Willebrand syndrome in patients with<br />
ventricular assist device or total artificial heart”<br />
Authors: C. Heilmann, U. Geisen, F. Beyersdorf, L. Nakamura, C. Benk, M. Berchtold-Herz,<br />
G. Trummer, C. Schlensak, B. Zieger; University Medical Center Freiburg, Freiburg, Germany<br />
Published in Thrombosis and Haemostasis 2010 103 5: 962-967<br />
Summary:<br />
“Unexplained bleeding episodes are associated with ventricular assist devices (VAD) and can occur in part due to<br />
acquired von Willebrand syndrome (AVWS)… We studied 12 patients who required mechanical support of their<br />
native heart for terminal cardiac insufficiency. Nine patients underwent placement of a VAD, while three<br />
underwent placement of a total artificial heart (TAH)…<br />
AVWS was present within two weeks of implantation in eight of nine patients… AVWS was not observed in the<br />
TAH patients studied. Our findings demonstrate that patients with an implanted VAD experience a rapid onset<br />
of AVWS that is quickly and completely reversed after device explantation.”<br />
Prof. Dr. Friedhelm<br />
Beyersdorf, Director of<br />
Cardiovascular Surgery<br />
at University Medical<br />
Center Freiburg,<br />
Germany
First of 3<br />
consecutive pediatric<br />
patients bridged to transplant<br />
Jordan Merecka, 18, was the first pediatric <strong>SynCardia</strong> Total Artificial Heart patient to<br />
be discharged from Texas Children’s Hospital to wait for a matching donor heart at<br />
home using the Freedom® portable driver. Jordan, pictured with his mother Suzanne, received<br />
his donor heart transplant on Oct. 29, 2011, after 160 days of life with the Total Artificial Heart.<br />
<strong>SynCardia</strong> Drivers Power the Total Artificial Heart<br />
The Companion 2 “launch” driver powers the Total Artificial Heart in the hospital from the operating room implant until the patient’s<br />
condition stabilizes. Stable patients who are eligible are then switched to the Freedom® portable driver for in-hospital ambulation<br />
and home discharge*.<br />
Companion 2 Hospital Implant Driver<br />
CE approved for use in Europe<br />
FDA approved for use in the U.S.<br />
The Companion 2 driver can be docked in the Hospital Cart during patient recovery<br />
and the Caddy for in-hospital ambulation.<br />
Freedom® Portable Driver<br />
CE approved for discharge in Europe<br />
April 2012: Enrollment completed in FDA-approved<br />
Investigational Device Exemption (IDE) clinical study<br />
n World’s first wearable power supply for the Total Artificial Heart<br />
Design Features:<br />
n Runs on hospital air for quieter operation<br />
n Runs on internal compressors for improved mobility<br />
n Longer service life<br />
User-friendly touchscreen provides access to<br />
all driver functions… no keyboard required<br />
Three Operating Modes<br />
n Weighs 13.5 lbs ( ~6 kg) including two onboard rechargeable batteries<br />
n Designed to be carried by the patient in the Freedom Backpack or Shoulder Bag<br />
n Batteries are charged using a standard electrical outlet or<br />
car cigarette lighter adaptor<br />
*CAUTION – The Freedom® portable driver is an investigational device,<br />
limited by United States law to investigational use.<br />
Companion 2 and Freedom drivers are serviced by replacement<br />
n No on-site repair required<br />
n No inventory of parts required<br />
Jordan Merecka<br />
UNITED STATES<br />
Implanted: May 22, 2011<br />
Freedom® discharge: Aug. 31, 2011<br />
Transplanted: Oct. 29, 2011<br />
n Operating Room – all features and functions are available<br />
n Intensive Care Unit – full access to alarm modes and<br />
monitoring of critical functions<br />
n Ambulatory – heart rate & drive pressures need no adjustment<br />
The Freedom® portable driver with the<br />
<strong>SynCardia</strong> temporary Total Artificial Heart<br />
French Total<br />
Artificial Heart<br />
patient Mr. Amoussou,<br />
pictured in September 2010,<br />
carries his Freedom® portable<br />
driver in the Shoulder Bag.<br />
Antonio Amoussou FRANCE<br />
Implanted: Feb. 16, 2010<br />
Freedom® discharge: July 5, 2010<br />
Transplanted: Jan. 2, 2011<br />
7
30 Years of Proven Reliability<br />
Longest supported Total Artificial Heart patient, 1,374 days<br />
(nearly 4 years) prior to transplant<br />
1,000+ implants of the Total Artificial Heart account for more than 270 patient years of life<br />
The valves in the Total Artificial Heart have never failed<br />
The diaphragm has a failure rate of less than 1% over 2,000+ diaphragms<br />
<strong>SynCardia</strong> Products Under Development<br />
CAUTION - The Permanent Total Artificial Heart, 50cc Total Artificial Heart and Freedom® 2 prototypes are not for human use. These products<br />
are in the research and development phase and will not be ready for sale in the U.S. or Europe until after all regulatory requirements have been met.<br />
Permanent Total Artificial Heart<br />
n Intended for patients with a Body Surface Area (BSA) of 1.7m 2 or greater<br />
n On March 2, 2012, the FDA approved a Humanitarian Use Device (HUD) designation<br />
for the Total Artificial Heart to be used on a permanent basis.<br />
n <strong>SynCardia</strong> is currently working on submitting a Humanitarian Device<br />
Exemption (HDE) to the FDA.<br />
n Once approved, the HDE will allow up to 4,000 U.S. patients annually who are not<br />
transplant-eligible to receive the Total Artificial Heart on a permanent basis.<br />
New 50cc Total Artificial Heart<br />
n Intended for patients with a BSA of 1.2 to 1.7m 2<br />
n Designed for women, men of smaller stature and adolescents<br />
n Together, the 70cc and 50cc Total Artificial Hearts are designed to fit almost all<br />
adult men and women, and most adolescents.<br />
Freedom® 2 Portable Driver (In-hospital ambulation & home discharge)<br />
Intended Design Features:<br />
n Lighter and easier to carry<br />
n Quieter operation for improved quality of life<br />
n Longer service life<br />
For information about becoming a <strong>SynCardia</strong> Certified Center, contact:<br />
World’s Longest Supported Total Artificial Heart Patient<br />
Mr. Zorzetto, wearing the Freedom® portable driver in the Backpack, is the world’s longest supported<br />
Total Artificial Heart patient at 1,374 days. He received a matching donor heart after nearly 4 years of support.<br />
www.syncardia.com<br />
Pietro Zorzetto ITALY<br />
Implanted: Dec. 6, 2007<br />
Discharged: Feb. 4, 2008<br />
Transplanted: Sept. 9, 2011<br />
70cc <strong>SynCardia</strong> Total Artificial Heart<br />
50cc <strong>SynCardia</strong> Total Artificial Heart<br />
Freedom® 2 Portable Driver<br />
VP of Global Certification & Logistics Managing Directors<br />
Mary Pat Sloan +1 520 440-7593 | msloan@syncardia.com Dr. Oliver Voigt & Markus Leinberger | europe@syncardia.com<br />
1992 East Silverlake Rd. | Tucson, AZ 85713 +49 700 SYNCARDIA (796227342)