Proceedings - Interdisciplinary Center for Nanotoxicity
Proceedings - Interdisciplinary Center for Nanotoxicity
Proceedings - Interdisciplinary Center for Nanotoxicity
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Conference on Current Trends in Computational Chemistry 2009<br />
Mechanisms of 1,2‐Cycloadditions of 1,3‐Imidazol‐2‐ylidene<br />
to 4‐Substituted Phenylethenes<br />
Fillmore Freeman* and Tieuphuong Dinh Le<br />
Department of Chemistry, University of Cali<strong>for</strong>nia, Irvine, Irvine, Cali<strong>for</strong>nia 92697<br />
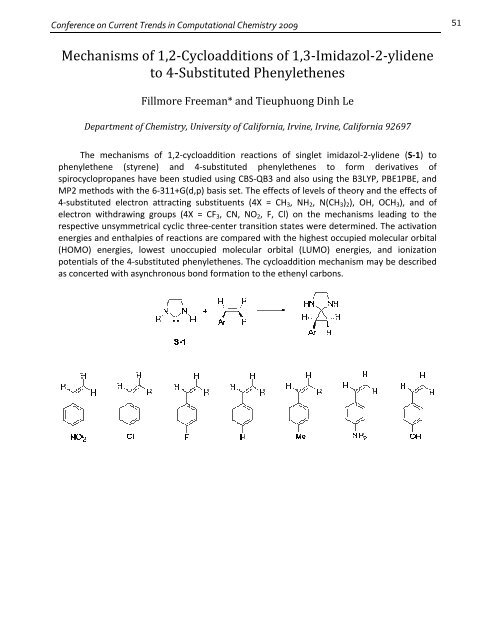
The mechanisms of 1,2‐cycloaddition reactions of singlet imidazol‐2‐ylidene (S‐1) to<br />
phenylethene (styrene) and 4‐substituted phenylethenes to <strong>for</strong>m derivatives of<br />
spirocyclopropanes have been studied using CBS‐QB3 and also using the B3LYP, PBE1PBE, and<br />
MP2 methods with the 6‐311+G(d,p) basis set. The effects of levels of theory and the effects of<br />
4‐substituted electron attracting substituents (4X = CH3, NH2, N(CH3)2), OH, OCH3), and of<br />
electron withdrawing groups (4X = CF3, CN, NO2, F, Cl) on the mechanisms leading to the<br />
respective unsymmetrical cyclic three‐center transition states were determined. The activation<br />
energies and enthalpies of reactions are compared with the highest occupied molecular orbital<br />
(HOMO) energies, lowest unoccupied molecular orbital (LUMO) energies, and ionization<br />
potentials of the 4‐substituted phenylethenes. The cycloaddition mechanism may be described<br />
as concerted with asynchronous bond <strong>for</strong>mation to the ethenyl carbons.<br />
51