HIGHER CHEMISTRY - Oxidation - Education Scotland
HIGHER CHEMISTRY - Oxidation - Education Scotland
HIGHER CHEMISTRY - Oxidation - Education Scotland
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>HIGHER</strong><br />
<strong>CHEMISTRY</strong><br />
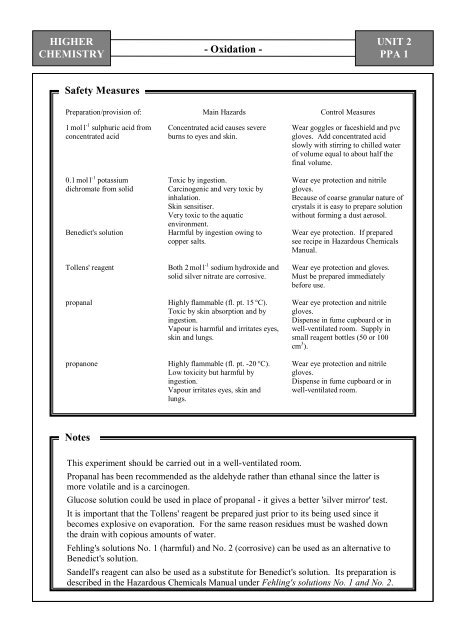
Safety Measures<br />
- <strong>Oxidation</strong> -<br />
Preparation/provision of: Main Hazards Control Measures<br />
1 mol l -1 sulphuric acid from<br />
concentrated acid<br />
0.1 mol l -1 potassium<br />
dichromate from solid<br />
Concentrated acid causes severe<br />
burns to eyes and skin.<br />
Toxic by ingestion.<br />
Carcinogenic and very toxic by<br />
inhalation.<br />
Skin sensitiser.<br />
Very toxic to the aquatic<br />
environment.<br />
Benedict's solution Harmful by ingestion owing to<br />
copper salts.<br />
Tollens' reagent Both 2 mol l -1 sodium hydroxide and<br />
solid silver nitrate are corrosive.<br />
propanal Highly flammable (fl. pt. 15 °C).<br />
Toxic by skin absorption and by<br />
ingestion.<br />
Vapour is harmful and irritates eyes,<br />
skin and lungs.<br />
propanone Highly flammable (fl. pt. -20 °C).<br />
Low toxicity but harmful by<br />
ingestion.<br />
Vapour irritates eyes, skin and<br />
lungs.<br />
Notes<br />
UNIT 2<br />
PPA 1<br />
Wear goggles or faceshield and pvc<br />
gloves. Add concentrated acid<br />
slowly with stirring to chilled water<br />
of volume equal to about half the<br />
final volume.<br />
Wear eye protection and nitrile<br />
gloves.<br />
Because of coarse granular nature of<br />
crystals it is easy to prepare solution<br />
without forming a dust aerosol.<br />
Wear eye protection. If prepared<br />
see recipe in Hazardous Chemicals<br />
Manual.<br />
Wear eye protection and gloves.<br />
Must be prepared immediately<br />
before use.<br />
Wear eye protection and nitrile<br />
gloves.<br />
Dispense in fume cupboard or in<br />
well-ventilated room. Supply in<br />
small reagent bottles (50 or 100<br />
cm 3 ).<br />
Wear eye protection and nitrile<br />
gloves.<br />
Dispense in fume cupboard or in<br />
well-ventilated room.<br />
This experiment should be carried out in a well-ventilated room.<br />
Propanal has been recommended as the aldehyde rather than ethanal since the latter is<br />
more volatile and is a carcinogen.<br />
Glucose solution could be used in place of propanal - it gives a better 'silver mirror' test.<br />
It is important that the Tollens' reagent be prepared just prior to its being used since it<br />
becomes explosive on evaporation. For the same reason residues must be washed down<br />
the drain with copious amounts of water.<br />
Fehling's solutions No. 1 (harmful) and No. 2 (corrosive) can be used as an alternative to<br />
Benedict's solution.<br />
Sandell's reagent can also be used as a substitute for Benedict's solution. Its preparation is<br />
described in the Hazardous Chemicals Manual under Fehling's solutions No. 1 and No. 2.