Masterpieces in Process Chemistry - The Scripps Research Institute
Masterpieces in Process Chemistry - The Scripps Research Institute
Masterpieces in Process Chemistry - The Scripps Research Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
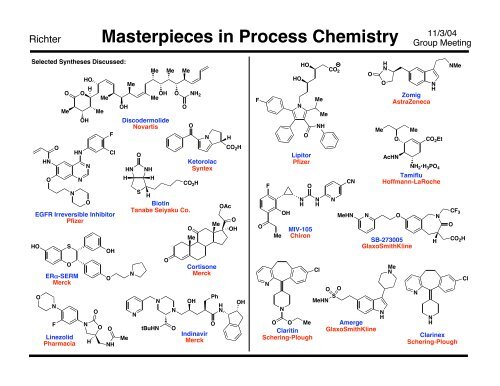
Richter<br />
Selected Syntheses Discussed:<br />
O<br />
Me<br />
O<br />
HO<br />
OH<br />
H<br />
Me<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
Me<br />
EGFR Irreversible Inhibitor<br />
Pfizer<br />
HO<br />
O<br />
HN<br />
O<br />
O<br />
S<br />
O<br />
N<br />
HN<br />
ERa-SERM<br />
Merck<br />
N<br />
F<br />
L<strong>in</strong>ezolid<br />
Pharmacia<br />
N<br />
N<br />
N<br />
O<br />
H<br />
O<br />
O<br />
O<br />
F<br />
Cl<br />
OH<br />
O<br />
NH<br />
OH<br />
Me<br />
Me<br />
OH<br />
Me<br />
Discodermolide<br />
Novartis<br />
HN<br />
N<br />
O<br />
NH<br />
H H<br />
Me<br />
Me<br />
O<br />
Me<br />
O<br />
NH 2<br />
O<br />
S<br />
H<br />
Biot<strong>in</strong><br />
Tanabe Seiyaku Co.<br />
N<br />
N<br />
tBuHN<br />
O<br />
N<br />
O<br />
N<br />
Ketorolac<br />
Syntex<br />
CO 2H<br />
O<br />
Me<br />
Me<br />
Cortisone<br />
Merck<br />
OH<br />
Ind<strong>in</strong>avir<br />
Merck<br />
Ph<br />
O<br />
H<br />
OAc<br />
H<br />
N<br />
CO 2H<br />
O<br />
OH<br />
OH<br />
F<br />
O<br />
F<br />
N<br />
Me<br />
OH<br />
N<br />
HO<br />
N<br />
HO<br />
O<br />
Lipitor<br />
Pfizer<br />
N<br />
H<br />
O<br />
MIV-105<br />
Chiron<br />
O O<br />
Clarit<strong>in</strong><br />
Me<br />
Scher<strong>in</strong>g-Plough<br />
Me<br />
Me<br />
NH<br />
N<br />
H<br />
Cl<br />
CO 2<br />
N<br />
O O<br />
S<br />
MeHN<br />
CN<br />
O<br />
Amerge<br />
GlaxoSmithKl<strong>in</strong>e<br />
Me<br />
H<br />
N<br />
O<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
N<br />
H<br />
Zomig<br />
AstraZeneca<br />
O<br />
AcHN<br />
Me<br />
CO 2Et<br />
NH 2•H 3PO 4<br />
Tamiflu<br />
Hoffmann-LaRoche<br />
MeHN N O<br />
N<br />
SB-273005<br />
GlaxoSmithKl<strong>in</strong>e<br />
N<br />
H<br />
Me<br />
N<br />
N<br />
N<br />
H<br />
H<br />
NMe<br />
CF 3<br />
O<br />
CO 2H<br />
Clar<strong>in</strong>ex<br />
Scher<strong>in</strong>g-Plough<br />
Cl
Richter<br />
Selected Syntheses Not Discussed:<br />
HO<br />
MeO<br />
Me<br />
F 3C<br />
O<br />
O<br />
AcO<br />
O<br />
O<br />
H<br />
NH<br />
HO<br />
H<br />
S H<br />
N<br />
OH<br />
Ecte<strong>in</strong>ascid<strong>in</strong> 743<br />
Corey<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
N Me<br />
H<br />
OMe<br />
O NHMe<br />
Prozac<br />
Eli Lilly<br />
MeN<br />
HO<br />
OH<br />
O N<br />
Cl<br />
Me<br />
FTI Candidate<br />
Pfizer<br />
N<br />
Me<br />
Cl<br />
Me<br />
H<br />
O<br />
CO 2Me<br />
O<br />
N N<br />
O<br />
Indoxacarb<br />
DuPont<br />
OH<br />
H<br />
N<br />
CO 2 –<br />
N<br />
CO 2Me<br />
Thienamyc<strong>in</strong><br />
Merck<br />
See Jeremy Richter, Baran<br />
Group Meet<strong>in</strong>g, January 2004<br />
H<br />
N<br />
N<br />
Zyprexa<br />
Eli Lilly<br />
S<br />
N<br />
Me<br />
NMe<br />
OCF 3<br />
NH 3 +<br />
HO<br />
HO<br />
HO<br />
H<br />
Disclaimers:<br />
O<br />
H<br />
O<br />
H<br />
Me H<br />
Me<br />
Me<br />
H H H H H H<br />
O O O<br />
O<br />
H<br />
Halichondr<strong>in</strong><br />
Kishi<br />
O<br />
H H<br />
O<br />
O<br />
O<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
O O<br />
H<br />
O<br />
Me<br />
1. This is by no means a comprehensive sampl<strong>in</strong>g of the many<br />
masterpieces <strong>in</strong> process chemistry.<br />
2. <strong>Process</strong> syntheses are very difficult to locate and decipher, s<strong>in</strong>ce most of<br />
the relevant literature is burried <strong>in</strong> patents and the words "process scale" do<br />
not appear <strong>in</strong> the titles.<br />
3. Some of the syntheses not discussed above were not done so because<br />
they were either not actual process routes (Ecte<strong>in</strong>ascid<strong>in</strong> 743, Halichondr<strong>in</strong>)<br />
or I was unable to locate the relevant literature <strong>in</strong> time.<br />
4. To give this topic the credit it deserves would require the publication of<br />
Classics <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong>.<br />
5. Many of the syntheses presented here are wonderfull full papers that<br />
del<strong>in</strong>eate the entire conception process along with problems encountered<br />
along the way. I recommend these papers for more <strong>in</strong>formation.<br />
Partial List of Transforms:<br />
Zhao olef<strong>in</strong>ation, Parikh-Doer<strong>in</strong>g oxidation, Still-Genari olef<strong>in</strong>ation, Nozaki-<br />
Hiyama coupl<strong>in</strong>g, Evans-Saksena reduction, Kagan oxidation, Ullmann<br />
reaction, Strecker reaction, Moffit oxidation, Fukuyama coupl<strong>in</strong>g, Wohl-<br />
Zeigler brom<strong>in</strong>ation<br />
H<br />
H<br />
H<br />
H<br />
H<br />
O<br />
O<br />
H<br />
O<br />
H<br />
H
Richter<br />
(–)-Discodermolide<br />
O<br />
Me<br />
O<br />
HO<br />
OH<br />
H<br />
Me<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
Me<br />
OH<br />
Me<br />
Me<br />
OH<br />
Me<br />
Me<br />
O<br />
Me<br />
O<br />
NH 2<br />
1. Non-taxane microtubule stabiliz<strong>in</strong>g agent (most potent known).<br />
2. Small amounts available naturally and must be harvested by manned<br />
submersibles. Fermentation has not been successful so all material<br />
must come from total synthesis.<br />
3. Currently <strong>in</strong> phase I cl<strong>in</strong>ical trials.<br />
4. Previous syntheses:<br />
a. Schreiber, JACS 1993, 115, 12621; ibid. 1996, 118, 11054<br />
b. Smith, JACS 1995, 117, 12011; OL 1999, 1, 1823; ibid. 2000, 2,<br />
1983; JACS 2000, 122, 8654<br />
c. Myles, JOC 1997, 62, 6098<br />
d. Marshall, JOC 1998, 63, 7885<br />
e. Paterson, ACIEE 2000, 39, 377; TL 2000, 41, 6935; JACS 2001,<br />
123, 9535; OL 2003, 5, 35.<br />
5. Novartis <strong>Process</strong> Synthesis:<br />
a. Drew heavily upon the Smith and Paterson approaches<br />
b. OPRD 2004, 8, 92-130<br />
"One major problem associated with a synthesis of this length is the proper laboratory<br />
exam<strong>in</strong>ation of the later reactions <strong>in</strong> a sequence. Initially, there are no answers to<br />
these supply problems; one just has to run the small-scale reaction and hope that on<br />
transfer to larger scale the reaction proceeds as expected. . . . On a positive note, this<br />
project was a first for Novartis, and its progress was avidly followed by the entire<br />
department who were all <strong>in</strong>terested <strong>in</strong> the "disco". <strong>The</strong> success of this project and its<br />
chemistry paves the way for other, perhaps even more complex, natural products to<br />
be prepared for early-phase cl<strong>in</strong>ical evaluations and sends a positive message to both<br />
the isolation and synthetic academic community and possibly other pharmaceutical<br />
companies that: "your work need not just be of academic <strong>in</strong>terest" and it may be<br />
worth tak<strong>in</strong>g a few risks. A total of 43 chemists participated <strong>in</strong> the concept of the<br />
synthesis, experimental design, and execution. . . . <strong>The</strong> hybridized Novartis–Smith–<br />
Paterson synthetic route that resulted <strong>in</strong> the preparation of 60 g of a structurally<br />
complex molecule conta<strong>in</strong><strong>in</strong>g 13 stereogenic centers is a crown<strong>in</strong>g achievement to all<br />
those who participated <strong>in</strong> this endeavor. <strong>The</strong> option of optimiz<strong>in</strong>g the present<br />
synthesis further or replac<strong>in</strong>g with a better one is a topic of our ongo<strong>in</strong>g studies, and<br />
we are confident of climb<strong>in</strong>g this mounta<strong>in</strong> as the situation demands."<br />
HO<br />
PMBO<br />
PMBO<br />
i. LiOH<br />
H 2O 2<br />
PMBO<br />
Me PMBO CCl 3<br />
CO 2Me<br />
Me<br />
Me<br />
Me<br />
OH<br />
ii.<br />
MeO<br />
OH<br />
OH<br />
Me<br />
N<br />
Me<br />
NH<br />
PPTS, DCM<br />
> 98%<br />
TEMPO,<br />
Bleach,<br />
DCM<br />
100%<br />
[Swern not amenable<br />
for large scale – stench]<br />
O<br />
O<br />
Cl<br />
N<br />
X c<br />
N<br />
O<br />
OMe<br />
PMBO<br />
PMBO<br />
i. LiOH, H 2O 2, MeOH<br />
ii. (R)-Phenylethylam<strong>in</strong>e<br />
84%<br />
[First purification:<br />
crystallization]<br />
N OMe<br />
N N<br />
OMe<br />
MeNHOMe<br />
85%<br />
Me<br />
Me<br />
PMBO<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
CO 2Me<br />
O<br />
PMBO<br />
LiBH 4, THF<br />
> 98%<br />
[LAH worked well<br />
but filtration = 24 hrs]<br />
Me<br />
N O<br />
Bn<br />
Me<br />
O<br />
O<br />
Bu2BOTf, TEA, > 75%<br />
[46-55% on<br />
20-25 kg scale]<br />
Me<br />
OH<br />
Me<br />
Me<br />
O<br />
NH 3<br />
O<br />
i. HCl<br />
ii. i BuOCOCl<br />
iii. MeNHOMe<br />
75-80%<br />
OH<br />
Me<br />
O<br />
Me<br />
N<br />
OMe
Richter<br />
PMBO<br />
PMBO<br />
PMBO<br />
Me<br />
OH<br />
Me<br />
TBSO<br />
Me<br />
O<br />
Me<br />
NMeOMe<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
Me<br />
(15:1 cis:trans, 31%)<br />
[Chromatography required]<br />
[No larger than 2.5 kg]<br />
Me<br />
TBSO<br />
Me<br />
O<br />
I<br />
TBSOTf,<br />
2,6-Lut.,<br />
Tol., 0 ºC<br />
90%<br />
[Chromatographic<br />
purification – 12 kg]<br />
PMBO<br />
Me<br />
TBSO<br />
[DIBAL-H reduction worked<br />
but –78 ºC was unacceptable]<br />
[Chromatography required]<br />
NaHMDS, THF,<br />
I<br />
Name?<br />
Ph 3P Me<br />
NMeOMe i. H 2, Pd/C, t BuOH<br />
ii. TEMPO, PhI(OAc) 2<br />
I<br />
PMBO<br />
Me<br />
Me<br />
TBSO<br />
NaHMDS, I 2 Ph 3P Me<br />
Me<br />
O<br />
I<br />
Me<br />
TBSO<br />
Name?<br />
Me<br />
O TBSO<br />
O<br />
NMeOMe<br />
Red-Al,<br />
Tol., –20 ºC<br />
68%<br />
Me<br />
Me<br />
O<br />
O<br />
NMeOMe<br />
i. MeMgBr<br />
ii. SO 3, Py,<br />
DMSO<br />
Me<br />
O<br />
66% overall<br />
[Chromatography<br />
Required]<br />
NMeOMe<br />
PMBO<br />
O<br />
Me<br />
OH<br />
Me<br />
O<br />
i. TBSOTf,<br />
2,6-Lut, 100%<br />
ii. LiBH 4, THF<br />
–30 ºC to RT,<br />
60%<br />
Me<br />
O<br />
OMe<br />
Me<br />
Me<br />
OTBS<br />
NMeOMe DDQ, Tol.,<br />
O<br />
OH<br />
Me<br />
[Chromatography<br />
Required]<br />
O<br />
OMe<br />
Me<br />
M.S., 0 ºC<br />
61%<br />
OH<br />
Bn<br />
Me<br />
O<br />
N<br />
85%<br />
[Crystall<strong>in</strong>e]<br />
Ph 3P, I 2, imid.,<br />
Tol., RT, 90%<br />
O<br />
O<br />
O<br />
O<br />
Me<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
O<br />
OMe<br />
Me<br />
Me<br />
O<br />
NMeOMe<br />
i. LAH, THF<br />
ii. Bu 2BOTf, TEA,<br />
–78 ºC to –10 ºC,<br />
O<br />
OMe<br />
Bn<br />
Me<br />
Me<br />
O<br />
N<br />
Me<br />
OTBS<br />
24% overall<br />
O<br />
I<br />
O
Richter<br />
PMBO<br />
PMBO<br />
Me<br />
Me<br />
Name?<br />
MeO 2C<br />
TBSO<br />
TBSO<br />
Me<br />
Me<br />
I<br />
I<br />
Me<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
Me<br />
Me<br />
Me<br />
TBSO O O<br />
i. DIBAL–H, 92%<br />
ii. SO 3, Pyr.,<br />
DMSO, 93%<br />
Me<br />
Me<br />
Me<br />
OTBS OPMB<br />
Me<br />
i. DDQ, H 2O, 88%<br />
ii. PhI(OAc) 2, TEMPO<br />
iii. KHMDS, 18-c-6, 76%<br />
O<br />
F3CH2CO F3CH2CO P CO2Me Me<br />
TBSO<br />
Me<br />
Me<br />
Me<br />
O<br />
PMBO<br />
Me<br />
OMe<br />
Me<br />
PMBO<br />
Me<br />
TBSO<br />
OTBS OH<br />
Me<br />
[Chromatography<br />
required]<br />
i. t BuLi, 9-MeOBBN,<br />
THF, –78 ºC<br />
ii. Cs 2CO 3, DMF,<br />
Pd(dppf) 2Cl 2, RT<br />
TBSO<br />
Me<br />
Me<br />
i. CrCl 2,<br />
Br<br />
TMS<br />
ii. KOH<br />
Me<br />
Me<br />
Me<br />
Me<br />
OTBS O O<br />
Me<br />
Name?<br />
Me<br />
Me<br />
OTBS OPMB<br />
Me<br />
81%<br />
[Chromatography<br />
required]<br />
i. CCl 3CONCO;<br />
Na 2CO 3, MeOH, 100%<br />
OMe<br />
ii. DIBAL–H, DCM, –78 ºC<br />
iii. PhI(OAc) 2, TEMPO, 80%<br />
O<br />
Me<br />
TBSO<br />
Me<br />
Me<br />
Me<br />
[problems with commercial<br />
purity of (+)-DIP-Cl]<br />
MeOMeN<br />
MeOMeN<br />
O<br />
Me<br />
Me<br />
O TBSO<br />
Me<br />
O TBSO<br />
O<br />
HO<br />
OH<br />
H<br />
Me<br />
+<br />
OTBS OCONH2 Me<br />
[Chromatography<br />
required]<br />
HO<br />
Me<br />
HO<br />
Me<br />
Me<br />
Me<br />
(+)-DIP-Cl, TEA, 55%<br />
Me<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
Me<br />
O TBSO<br />
Me<br />
MeOMeN Me<br />
Me<br />
Me<br />
OTBS<br />
Me<br />
Me<br />
OCONH 2<br />
O TBSO 4:1 dr, recyclable<br />
[Chromatography required<br />
on reverse-phase silica]<br />
Name?<br />
Me<br />
OH<br />
Me<br />
OH<br />
TBSO<br />
Me<br />
Me 4N + (OAc) 3BH, 73%<br />
Me<br />
HCl, MeOH<br />
Me<br />
OH<br />
Me<br />
Me<br />
Me<br />
OTBS<br />
Me<br />
Me<br />
O<br />
discodermolide<br />
[ > 60 g produced]<br />
Me<br />
O<br />
Me<br />
OCONH 2<br />
O<br />
[Chromatography<br />
required]<br />
NH 2<br />
39 steps, 17 chromatographic purifications, 20 months<br />
7 problematic steps identified and be<strong>in</strong>g optimized
F<br />
Richter<br />
EGFR Irreversible Inhibitor<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
HN<br />
O<br />
O<br />
1. Treatment of solid tumors.<br />
2. Inhibits Epidermal Growth Factor Tyros<strong>in</strong>e K<strong>in</strong>ase.<br />
3. <strong>Process</strong> synthesis – Rober Hughes, Pfizer, Gordon <strong>Research</strong><br />
Conference Presentation.<br />
O 2N<br />
Initial Route Problems Improved Route<br />
F<br />
CO 2H<br />
NH 2<br />
O<br />
N<br />
NH<br />
[6.5:1 regioselectivity]<br />
Could not improve<br />
NH<br />
H NH 2<br />
•AcOH<br />
CH3OCH2CH2OH 98%<br />
N<br />
HN<br />
F<br />
N<br />
i. SOCl 2, 98%<br />
ii. TEA, i PrOH,<br />
H 2N<br />
F<br />
N<br />
O<br />
O<br />
N<br />
F<br />
Cl<br />
O 2N<br />
NH<br />
i. 65% HNO 3/<br />
H 2SO 4, 70 ºC, 81%<br />
ii. HOAc, 57%<br />
[Yield loss]<br />
HN<br />
Cl F N<br />
Used DMF <strong>in</strong>stead of<br />
HOAc for Recrystallization<br />
74%<br />
N<br />
F<br />
Cl<br />
86%<br />
O<br />
N OH<br />
KOtBu, THF<br />
98%<br />
O2N [83% after recrystallization] O N<br />
Comb<strong>in</strong>ed 3 operations<br />
<strong>in</strong>to one pot, 95%<br />
Ra-Ni, THF<br />
H 2, 99%<br />
[Observed losses to dechlor<strong>in</strong>ation]<br />
1% Pt/C, THF, H 2<br />
80% from EtOH<br />
< 0.2% deschloro<br />
[Material still lost <strong>in</strong> cyrstalization]<br />
COCl<br />
TEA, EtOAc,<br />
51%<br />
[2 recrystallizations]<br />
Last step was optimizable, but for<br />
legal reasons they had to develop:<br />
i. Ac 2O, 85%<br />
ii. 1.5% Pt/C, H 2, THF, 99%<br />
H 2N<br />
O<br />
HN<br />
COCl<br />
Cl<br />
iii. TEA, THF, 0 ºC; NaOH, 80%<br />
iv. MeSO3H/AcOH/THF; NaOH, 90%<br />
O<br />
O<br />
N<br />
N<br />
HN<br />
N<br />
HN<br />
N<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
N<br />
HN<br />
N<br />
O<br />
N<br />
O<br />
N<br />
O<br />
F<br />
F<br />
Cl<br />
Cl<br />
F<br />
Cl<br />
F<strong>in</strong>al:<br />
8 steps (3 pots)<br />
55% overall yield<br />
produced multikilo's
Richter<br />
Ketorolac<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
N<br />
CO 2H<br />
1. Non-steroidal anti<strong>in</strong>flammatory drug (NSAID).<br />
2. Powerful anti<strong>in</strong>flammatory and analgesic activity.<br />
3. 10 mg equiefficacious with morph<strong>in</strong>e (10 mg) for post-operative pa<strong>in</strong>.<br />
4. 10 mg equiefficacious with aspir<strong>in</strong> (650 mg) for postpartumpa<strong>in</strong>.<br />
5. 10 mg equiefficacious with acetam<strong>in</strong>ophen (1 g) or acetom<strong>in</strong>ophen<br />
(600 mg)/code<strong>in</strong> (60 mg) comb<strong>in</strong>ation.<br />
6. Syntex development: Muchowski, Adv. Med. Chem. 1992, 1, 109.<br />
1 st Generation Route<br />
H<br />
N<br />
O<br />
i. mCPBA<br />
ii. MeOH, HCl<br />
i. NCS, DMS<br />
DCM, –30 ºC<br />
ii. D, 60%<br />
Mechanism?<br />
H<br />
N<br />
SMe<br />
O<br />
H<br />
N<br />
NaH, DMF;<br />
H<br />
SMe<br />
55 ºC,<br />
O O<br />
MeO 2C<br />
N<br />
O O<br />
CO 2Me<br />
SO 2Me<br />
PhCONMe 2<br />
POCl 3, DCE<br />
O<br />
D<br />
O<br />
N<br />
O<br />
SMe<br />
i. NaH, DMF, 85 ºC<br />
ii. NaOH<br />
O<br />
O<br />
Ketorolac,<br />
21% from pyrrole<br />
racemic<br />
2 nd Generation Route<br />
O<br />
O<br />
H<br />
N<br />
O<br />
N<br />
Br<br />
i. HO –<br />
ii. H 2, Pd/C,<br />
MgO<br />
iii. HCl<br />
O<br />
Br<br />
Br 2<br />
0 ºC<br />
O<br />
O<br />
i. MeOH, HCl<br />
3 rd Generation Route<br />
Beg<strong>in</strong>s from Pyrrole and proceeds <strong>in</strong> 45% overall yield:<br />
See US Patent 6,197,976<br />
O<br />
ii. NaH, DMF, 75 ºC<br />
H<br />
N<br />
Ketorolac,<br />
47% from benzoyl pyrrole<br />
racemic<br />
Br<br />
Br<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
O<br />
DMF, 80 ºC<br />
O O<br />
N<br />
O O<br />
New <strong>Chemistry</strong> Discovered:<br />
1. Selective substitution of pyrrole at C–3 when protected as N–Silyl.<br />
2. Acid <strong>in</strong>duced isomerization of C–2 substituted pyrroles to C–3.<br />
3. New routes to pyrrole-2-carboxaldehydes.<br />
4. New routes to acylpyrroles.<br />
5. Mild reduction of acylpyrroles to alkylpyrroles.<br />
6. Conversion of acylpyrroles to acylpyrrolid<strong>in</strong>es.<br />
7. First reported <strong>in</strong>tramolecular carbenoid addition to a pyrrole nucleus.<br />
Br<br />
CO 2Me<br />
CO 2Me
Richter<br />
ERa-SERM<br />
HO<br />
S<br />
O<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
1. SERM = selective estrogen receptor modulator.<br />
2. Potentially useful for the treatment of bone loss, cartilage degeneration,<br />
endometriosis, uter<strong>in</strong>e fibroid disease, hot flashes, <strong>in</strong>creased levels of<br />
low-density lipoprote<strong>in</strong> cholesterol, cardiovascular disease, obesity,<br />
<strong>in</strong>cont<strong>in</strong>ence and cancer.<br />
3. Synthesis: Merck, PNAS, 2004, 101, 5776.<br />
O<br />
Me OMe<br />
N<br />
O<br />
O<br />
O<br />
80%<br />
(H 2N) 2CS,<br />
HCl, 92%<br />
Mechanism?<br />
I BnO<br />
I<br />
+<br />
OBn<br />
MgBr<br />
HO<br />
PhMe 3N + Br 3 –<br />
DME, 100%<br />
OH<br />
O<br />
Br<br />
O<br />
S<br />
O<br />
N<br />
O<br />
I<br />
i. BnBr, NaI,<br />
K 2CO 3, 84%<br />
ii. NaOH<br />
OBn<br />
BnO SH<br />
88%<br />
OH<br />
BnO<br />
BnO<br />
S<br />
O<br />
OH<br />
D-DIPT<br />
Ti(O i Pr) 4<br />
Cumene<br />
hydroperoxide<br />
Name?<br />
HO<br />
S<br />
O<br />
S<br />
O<br />
TMSI<br />
81%<br />
BnO<br />
OBn<br />
I<br />
OBn<br />
I<br />
OH<br />
O<br />
PhPOCl 2<br />
MeCN, 90%<br />
O –<br />
S +<br />
O<br />
BnO<br />
OBn<br />
CuI, K 2CO 3,<br />
2,2'-bipyridyl, 140 ºC<br />
BnO<br />
N<br />
N<br />
I<br />
OH<br />
S<br />
O<br />
92%<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
S<br />
O<br />
BH 3•THF<br />
10 ºC<br />
88%, 99% ee<br />
Mechanism?<br />
OBn<br />
O<br />
ERa-SERM<br />
8 steps<br />
37% overall yield<br />
N<br />
OBn<br />
I
Richter<br />
Biot<strong>in</strong><br />
HN<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
S<br />
NH<br />
H H<br />
H<br />
CO 2H<br />
1. Important <strong>in</strong> human nutrition and animal health.<br />
2. > 80 tons produced synthetically anually.<br />
3. Synthesis: Tanabe Seiyaku Co., Chem. Eur. J. 2004, ASAP.<br />
4. For a comprehensive review of Biot<strong>in</strong> syntheses see:<br />
Ryan Shenvi, Baran Lab Group Meet<strong>in</strong>g, July 2003.<br />
HS<br />
S<br />
O<br />
NH2•HCl H<br />
CO 2H<br />
NBn<br />
H<br />
O<br />
86%, >99% ee<br />
i. H 2O 2, K 2CO 3,<br />
DMSO/DCM<br />
ii. H 2O, filter<br />
iii. HCl<br />
i. PhOCOCl, NaOH,<br />
H 2O, Tol., RT<br />
ii. BnCl, NaOH,<br />
DMSO, H 2O, RT<br />
i. NaHSO 3, EtOAc<br />
ii. BnNH 2, DCM;<br />
NaCN, 8–20 ºC;<br />
NaHSO 3, NaCN<br />
S<br />
O<br />
H 2NOC<br />
Name?<br />
NBn +<br />
H<br />
NHBn<br />
S<br />
O<br />
S NBn<br />
i. NaBH4, H2SO4, THF, D<br />
H ii. DCC, TFA, Pyr.,<br />
CO2H DMSO, EtOAc,<br />
83%, >99% ee 50 ºC<br />
Name?<br />
O<br />
S<br />
O<br />
H<br />
93% 2NOC<br />
7%<br />
NHBn +<br />
H<br />
NHBn<br />
NC<br />
S<br />
O<br />
NC<br />
100%<br />
11:1 syn/anti<br />
NHBn DMF<br />
H<br />
NHBn<br />
120 ºC<br />
BnN<br />
NHBn<br />
H<br />
NHBn<br />
O<br />
S<br />
91%<br />
NBn<br />
H H<br />
O<br />
S<br />
O<br />
DMF, 90 ºC;<br />
NBn<br />
H HCl, 90 ºC<br />
NHBn 95%<br />
H 2NOC<br />
I<br />
IZn<br />
Zn<br />
Pd/C, THF,<br />
Tol, DMF<br />
94%<br />
CO 2Et<br />
CO 2Et<br />
i. H 2 (0.9 MPa), Pd(OH) 2/C,<br />
MeOH, H 2O;<br />
NaOH, 90%<br />
ii. MeSO 3H, mesitylene, 74%<br />
BnN<br />
O<br />
NBn<br />
H H<br />
Name?<br />
SH CO 2H<br />
BnN<br />
O<br />
S<br />
NBn<br />
H H<br />
HN<br />
O<br />
S<br />
NH<br />
H H<br />
H<br />
biot<strong>in</strong><br />
12 steps,<br />
39% overall<br />
DCC,<br />
TFA, Pyr.,<br />
10–60 ºC<br />
93%<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
CO 2Et<br />
CO 2H<br />
BnN<br />
O<br />
S<br />
NBn<br />
H H<br />
O
Richter<br />
Cortisone<br />
O<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
Me<br />
1. Synthesis at the time was meant to provide large quantities to test.<br />
2. Start<strong>in</strong>g material readily available from cow bile.<br />
3. Work done <strong>in</strong> the early 1950's without modern spectroscopy.<br />
4. Work done <strong>in</strong> less than 2 years.<br />
5. <strong>Process</strong> synthesis: Merck, OPRD, 2004, 8, 708.<br />
HO<br />
HO<br />
Me<br />
Me<br />
OH<br />
Me<br />
i. EtOCOCl, Pyr.<br />
ii. CrO 3<br />
Me<br />
O<br />
EtO 2CO<br />
Me<br />
Me<br />
91%<br />
Me<br />
CO 2H MeOH<br />
Me<br />
H 2SO 4<br />
O<br />
OAc<br />
Me<br />
Me<br />
O<br />
OH<br />
HO<br />
CO 2H i. MeOH, H +<br />
ii. H 2, Pt 0<br />
Me<br />
Me<br />
OH<br />
Me<br />
CO 2Me<br />
i. Br 2, MeOH/PhH<br />
ii. NaOAc, DMF<br />
CO2Me iii. HO – ; iv. H +<br />
95.5%<br />
SeO2 worked <strong>in</strong> 1 step<br />
but needed <strong>in</strong> Korean<br />
War electronics<br />
HO<br />
Me<br />
94%<br />
Me<br />
OH<br />
Me<br />
CO 2Me<br />
HO<br />
Me<br />
i. Br 2, CHCl 3<br />
Me<br />
OH<br />
Me<br />
ii. Na 2Cr 2O 7<br />
CrO 3, acetone<br />
O<br />
Me<br />
O<br />
NBS, PhH,<br />
hn; D<br />
Name?<br />
Me<br />
Me<br />
Ph<br />
CO 2Me<br />
Ph<br />
O<br />
Me<br />
O<br />
i. HBr,<br />
CHCl 3<br />
ii. H 2O,<br />
CHCl 3<br />
Me<br />
Br<br />
Me<br />
i. HBr<br />
CO 2Me<br />
75.5%<br />
61.6% from SM<br />
Me<br />
O<br />
Me<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
Me<br />
Me<br />
i. PhMgCl<br />
ii. HOAc, D<br />
Me<br />
Br<br />
Me<br />
CO 2Me<br />
ii. Ac<br />
87% 2O<br />
AcO<br />
92.7%<br />
AcO<br />
"As <strong>in</strong>terest<strong>in</strong>g as was the<br />
k<strong>in</strong>etics of acetic acid formation<br />
dur<strong>in</strong>g enol acetylation or<br />
peracid uptake dur<strong>in</strong>g the<br />
oxidation and despite the nice<br />
data plots, they taught little<br />
about m<strong>in</strong>or byproducts or overreaction."<br />
O<br />
Me<br />
AcO<br />
Me<br />
Br<br />
Me<br />
Ph<br />
87.4%<br />
O<br />
Me<br />
Ph<br />
Me<br />
Me<br />
O<br />
92%<br />
O<br />
Ph<br />
i. Na 2Cr 2O 7,<br />
H 2SO 4<br />
ii. Zn, HOAc<br />
DNBS, Ac 2O;<br />
MPPA; NaOH<br />
Ph<br />
[DNBS = d<strong>in</strong>itrobenzenesulfonic acid]<br />
[MPPA = monoperphthalic acid]
Richter<br />
HO<br />
O<br />
Me<br />
Me<br />
Me<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
OH i. Br 2, MeOH, PhH<br />
ii. KOAc, HOAc, NaI<br />
iii. DDH, acetone<br />
iv. Zn, HOAc<br />
[DDH = dibromodimethylhydanto<strong>in</strong>]<br />
"With benzene, we actually considered it beneficent <strong>in</strong> that carbon tetrachloride was a<br />
known liver tox<strong>in</strong>. Little did we know at the time that we were exchang<strong>in</strong>g it for what<br />
would many years later be labeled a carc<strong>in</strong>ogen!"<br />
i. Br 2, CHCl 3,<br />
HOAc<br />
ii. NaBr,<br />
acetone<br />
[step 2 used to convert<br />
all side products to the<br />
desire product]<br />
H 2NOCHNN<br />
94%<br />
O<br />
Me<br />
O<br />
AcO<br />
Br<br />
Me<br />
O<br />
Me<br />
O<br />
OH<br />
AcO<br />
Me<br />
95%<br />
HCl<br />
CHCl 3<br />
O<br />
OH<br />
O<br />
O<br />
O<br />
Me<br />
Me<br />
O<br />
OH<br />
86%<br />
52.6% from ether<br />
H 2NCONHNH 2<br />
O<br />
Me<br />
AcO<br />
"A great deal of development was still required as the demonstration with an<br />
<strong>in</strong>completely developed process was <strong>in</strong>itiated <strong>in</strong> the new plant. Some improvements<br />
were made on an ad-hoc basis, at times prematurely, with production at sub-optimal<br />
performance better than no production at all. For better and for worse, such a modus<br />
operandi is no longer practiced, courtesy of FDA and cGMP regulations."<br />
AcO<br />
Me<br />
O<br />
OH<br />
92%<br />
87.4% from triketone<br />
28.3% overall<br />
"Product elegance has long been an ethereal objective of ethical pharmaceutical<br />
companies; it is sometimes an expensive one. Plann<strong>in</strong>g for the last step has to <strong>in</strong>clude<br />
concerns of color and appearance as well as chemical purity. It is annoy<strong>in</strong>g to some<br />
synthetic chemists to see a difficultly won, elegant, white crystall<strong>in</strong>e material subjected<br />
by pharmacists to granulation, sometimes coloration, and compression to an unnatural<br />
form."<br />
L<strong>in</strong>ezolid<br />
O<br />
N<br />
F<br />
N<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
1. Active aga<strong>in</strong>st gram-positive and gram-negative bacteria with potency<br />
<strong>in</strong> the 2-4 mg/mL.<br />
2. Synthesis: Pharmacia/Pfizer, ACIEE, 2003, 42, 2010,<br />
US Patent: 5837870.<br />
O<br />
TEA,<br />
NsCl<br />
F<br />
F NO 2<br />
N<br />
F<br />
90% Overall<br />
O<br />
N<br />
F<br />
NHCbz<br />
85% Overall<br />
N<br />
i.<br />
O<br />
NH<br />
ii. Pd/C,<br />
H 2<br />
H<br />
i.<br />
H<br />
ii. KO t Bu<br />
iii. LDA<br />
O<br />
O<br />
O<br />
O<br />
HO H<br />
Cl OH<br />
O<br />
ONs<br />
O<br />
NH<br />
N<br />
F<br />
Me<br />
O<br />
i. NH 4OH,<br />
MeOH, 45 ºC<br />
ii. Ac 2O, 85%<br />
N<br />
F<br />
NH 2<br />
N<br />
CbzCl,<br />
K 2CO 3<br />
H<br />
O<br />
O<br />
l<strong>in</strong>ezolid<br />
9 steps<br />
65% overall yield<br />
OH
Richter<br />
Ind<strong>in</strong>avir<br />
N<br />
N<br />
tBuHN<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
1. HIV Protease Inhibitor.<br />
2. Synthesis: Merck, Chimia, 1997, 51, 306.<br />
Narendra Ambhaikar, Baran Group Meet<strong>in</strong>g, July 2004.<br />
N<br />
O<br />
(S,S)-Mn II (salen)Cl<br />
NaOCl, P 3NO<br />
enzyme<br />
i PrOAc,<br />
NaOH, 70 ºC;<br />
MsOH, 35 ºC,<br />
OAc<br />
Me<br />
Me Me<br />
Ph<br />
O<br />
N<br />
O<br />
88%<br />
COCl<br />
99% ee<br />
>50% yield<br />
Br<br />
LHMDS,<br />
–15 ºC<br />
94%<br />
OH<br />
NH 2<br />
OH<br />
Ph<br />
O<br />
H<br />
N<br />
87% ee<br />
OH<br />
99% ee<br />
O<br />
OH<br />
OH<br />
Tartaric acid,<br />
Ph Me Me<br />
O<br />
N<br />
base<br />
O<br />
Mechanism?<br />
Oleum,<br />
MeCN,<br />
H 2O<br />
NH 2<br />
i. NIS, H 2O,<br />
NaHCO 3, 91%<br />
ii. NaOMe, 100%<br />
OH<br />
N<br />
O<br />
CN<br />
N<br />
i. t BuOAc,<br />
H 2SO 4, 90%<br />
ii. H 2, Pd(OH) 2,<br />
95%<br />
Ph Me Me<br />
O<br />
N<br />
O<br />
N<br />
HN<br />
N<br />
tBuHN<br />
N<br />
tBuHN<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
NH<br />
CONHt i. pyroglutamic acid resolution<br />
with recycle, 47%<br />
ii. Boc2O, KOH, 80%<br />
Bu<br />
i. MeOH, D<br />
ii. HCl (g)<br />
N<br />
O<br />
N<br />
O<br />
OH<br />
94%<br />
OH<br />
Ph<br />
O<br />
N<br />
H2SO4 Ph<br />
O<br />
<strong>in</strong>d<strong>in</strong>avir<br />
75% over 3 steps<br />
>32% overall<br />
H<br />
N<br />
Cl<br />
H<br />
N<br />
BocN<br />
tBuHN<br />
OH<br />
OH<br />
N<br />
O
Richter<br />
Lipitor<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
1. Hypolipidemic.<br />
2. Number one sell<strong>in</strong>g drug of all time (Natural product <strong>in</strong>spired).<br />
3. Synthesis: Bruce Roth, Pfizer, Prog. Med. Chem. 2002, 40, 1.<br />
4. Largest competitors:<br />
Me<br />
Me<br />
Me<br />
O<br />
Me<br />
O<br />
HO<br />
H<br />
F<br />
Zocor<br />
Me<br />
Me<br />
O<br />
Me<br />
Me<br />
O<br />
O<br />
HO<br />
O<br />
N<br />
HO<br />
HO<br />
H<br />
O<br />
Mevacor<br />
Me<br />
Me<br />
Me<br />
NH<br />
O<br />
Me<br />
Me<br />
HO<br />
CO 2<br />
O<br />
O<br />
O<br />
H<br />
HO<br />
Pravacol<br />
HO<br />
Me<br />
CO 2Na<br />
HO<br />
Br<br />
NC<br />
HO<br />
OH<br />
OH<br />
Br<br />
O<br />
OTBS<br />
OH<br />
CONHPh<br />
Me<br />
O<br />
O<br />
CO 2Me<br />
CO 2Me<br />
i. NaBH 4, Et 2BOMe,<br />
MeOH, –90 ºC<br />
ii. Me 2C(OMe) 2,<br />
MeSO 3H, 65%<br />
Me<br />
H 2O 2,<br />
CaCO 3,<br />
K 2CO 3<br />
H 2,<br />
Pd/C<br />
i. NaOH<br />
ii. CDI,<br />
F<br />
HO<br />
O<br />
Br<br />
OH<br />
OH<br />
Mg(O t<br />
2CCH2CO2 Bu)2<br />
iii. TBAF, HOAc, THF<br />
or:<br />
3 equiv. LiCH t<br />
2CO2 Bu<br />
NC<br />
HO<br />
Me<br />
Me<br />
O O<br />
Mechanism?<br />
Me<br />
S<br />
Bn<br />
N +<br />
TEA<br />
Cl –<br />
OH<br />
CO 2 t Bu<br />
F<br />
CO 2K<br />
CO 2Me<br />
NC<br />
H 2N<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
HBr,<br />
HOAc,<br />
MeOH<br />
i. TBSCl, imid.,<br />
4-DMAP<br />
ii. NaCN, DMSO<br />
OH O<br />
H 2, Ra-Ni,<br />
MeOH, 95%<br />
O<br />
Me<br />
Me<br />
O O<br />
O<br />
Me<br />
CONHPh<br />
CO 2 t Bu<br />
Me<br />
CO 2 t Bu
Richter<br />
F<br />
O<br />
F<br />
F<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
Me<br />
CONHPh<br />
Me<br />
Me<br />
Me<br />
+<br />
O<br />
N<br />
HO<br />
N<br />
H 2N<br />
O<br />
O<br />
HO<br />
Me<br />
Me<br />
O O<br />
pivalic acid<br />
1:4:1 Tol.:heptane:THF<br />
D, 75%<br />
O<br />
Me<br />
Me<br />
NH<br />
CO 2 t Bu<br />
CO 2 t Bu<br />
"...produced stereochemically pure atorvastat<strong>in</strong> calcium <strong>in</strong> a convergent, commercially<br />
viable manner which accomplished the orig<strong>in</strong>al vision for the synthesis developed <strong>in</strong><br />
discovery chemistry, but was reduced to practice <strong>in</strong> chemical development."<br />
Me<br />
Me<br />
NH<br />
CO 2<br />
Zomig<br />
O<br />
H<br />
N<br />
NMe<br />
O<br />
N<br />
H<br />
1. Used to treat migra<strong>in</strong>e headaches.<br />
2. Synthesis: AstraZeneca, US Patent 6084103, 6160123,<br />
Li, J.J. Contemporary Drug Synthesis, Wiley, 2004.<br />
ClH•H 2N<br />
O<br />
MeO 2C<br />
H<br />
N<br />
NO 2<br />
i. NaNO 2, HCl, 0 ºC<br />
ii. Na 2SO 3, H 2O, 0–60 ºC<br />
iii. reflux, 3 hr<br />
OEt<br />
EtO<br />
NMe2 iv. 10% EtOH/EtOAc<br />
O<br />
N<br />
H<br />
No yields given<br />
One-pot procedure<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
i. Na 2CO 3, H 2O, EtOAc; ClCO 2Bu<br />
ii. H 2, 5% Pd/C, EtOAc, BuOH, 30–50 ºC<br />
iii. NaBH 4, BuOH, 35 ºC<br />
iv. NaOMe, MeOH, BuOH, 85 ºC<br />
NMe 2<br />
O<br />
H<br />
N<br />
O<br />
NH 2<br />
precipitated upon cool<strong>in</strong>g
Richter<br />
Tamiflu<br />
Me<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O<br />
AcHN<br />
Me<br />
CO 2Et<br />
NH 2•H 3PO 4<br />
1. Potent <strong>in</strong>hibitor of <strong>in</strong>fluenza neuram<strong>in</strong>idase at nanomolar concentrations.<br />
2. Synthesis: Hoffmann-LaRoche, Chimia, 2004, 58, 621.<br />
Me<br />
HO<br />
HO<br />
HO<br />
HO<br />
OH<br />
OH<br />
CO 2H<br />
OH<br />
CO2H i. NaN 3, NH 4Cl,<br />
DMF, 85 ºC<br />
ii. Ac 2O, Pyr.<br />
35%<br />
O<br />
AcHN<br />
Me<br />
N 3<br />
3 steps<br />
70–80%<br />
5 steps<br />
40–45%<br />
Me<br />
CO 2Et<br />
O<br />
HN<br />
Me<br />
Me<br />
O<br />
O<br />
Me<br />
CO 2Et<br />
i. L<strong>in</strong>dlar, H 2<br />
ii. H 3PO 4, 80%<br />
OMs<br />
CO 2Et<br />
i. NaN 3, NH 4Cl,<br />
EtOH, 65 ºC<br />
ii. PMe 3,<br />
MeCN, RT<br />
Me<br />
[A series of studies was undertaken to improve the<br />
efficiency and safety of this route, through the<br />
replacement of the azide chemistry, as well as<br />
beg<strong>in</strong>n<strong>in</strong>g with more cost effective start<strong>in</strong>g materials.]<br />
O<br />
AcHN<br />
Me<br />
Me<br />
i. Et 3SiH, TiCl 4<br />
ii. NaHCO 3<br />
80%<br />
O<br />
O<br />
Me<br />
CO 2Et<br />
NH 2•H 3PO 4<br />
CO 2Et<br />
Tamiflu<br />
35% from Shikimic acid<br />
20% from Qu<strong>in</strong>ic acid<br />
MIV-105<br />
O<br />
F<br />
OH<br />
Me<br />
1. Non-nucleoside reverse transcriptase <strong>in</strong>hibitor.<br />
2. Synthesis: Chiron, OPRD, 2004, 8, 353.<br />
F<br />
CO 2H<br />
OH<br />
i. HCl, dioxane, H 2O<br />
ii. LiOH, MeOH, H 2O<br />
iii. HCl<br />
O<br />
F<br />
OMe<br />
Me<br />
i. SOCl 2, DEA,<br />
0 ºC, 86%<br />
ii. BuMgN i Pr 2, THF, D;<br />
I 2, THF, 5 ºC, 56%<br />
N<br />
H<br />
O<br />
i. EtCOCl, Pyr., 100%<br />
ii. AlCl3, 88% Mechanism?<br />
iii. MeI, K 2CO 3, 97%<br />
iv. (CH 2OH) 2, pTSA,<br />
PhH, 86%<br />
CO 2H<br />
O<br />
F<br />
O<br />
OMe<br />
Me<br />
I<br />
N<br />
H<br />
O<br />
CO 2Et<br />
i. TEA, EtOCOCl<br />
ii. NaN 3; D<br />
iii.<br />
H 2N<br />
N<br />
CN<br />
O<br />
F<br />
O<br />
F<br />
N<br />
O<br />
CN<br />
NEt 2<br />
OMe<br />
Me<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
i. HNO3, D, 92%<br />
ii. IPA, BnHN Ph<br />
Me<br />
iii. HCl, 78%<br />
iv. TsOH, EtOH,<br />
D, 93%<br />
i. BuLi, THF, –78 ºC<br />
ii. ZnBr 2, –65 ºC<br />
iii. Pd(OAc) 2, (ArO) 3P,<br />
–65 ºC, 85%<br />
OMe<br />
Me<br />
Name?<br />
N<br />
H<br />
O<br />
I<br />
N<br />
H<br />
MIV-105<br />
27% overall yield<br />
N<br />
CO 2Et<br />
CN<br />
BCl 3, DCM, 52%
Richter<br />
Amerge<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
O O<br />
S<br />
MeHN<br />
N<br />
H<br />
Me<br />
N<br />
1. Used to treat migra<strong>in</strong>e headaches.<br />
2. Synthesis: GlaxoSmithKl<strong>in</strong>e, J. Med. Chem. 1995, 38, 3566,<br />
Li, J.J. Contemporary Drug Synthesis, Wiley, 2004.<br />
Br<br />
O O<br />
S<br />
MeHN<br />
N<br />
H<br />
O<br />
NMe<br />
KOH, IMS,<br />
D, 94%<br />
N<br />
H<br />
Me<br />
N<br />
Br<br />
H 2, Pd/C,<br />
DMF, H 2O,<br />
MeOH, 90%<br />
N<br />
H<br />
Me<br />
N<br />
i.<br />
O O<br />
S<br />
MeHN<br />
O O<br />
S<br />
MeHN<br />
Pd(OAc) 2, (o-tolyl) 3P,<br />
TEA, DMF, 85 ºC<br />
ii. HCl, 89%<br />
N<br />
H<br />
Amerge<br />
75% overall yield<br />
Me<br />
N<br />
Clarit<strong>in</strong> and Clar<strong>in</strong>ex<br />
N<br />
N<br />
O O Me<br />
1. Antihistam<strong>in</strong>es.<br />
2. Synthesis: Scher<strong>in</strong>g-Plough, J. Org Chem. 1989, 54, 2242,<br />
Li, J.J. Contemporary Drug Synthesis, Wiley, 2004.<br />
N<br />
N<br />
Me<br />
CN<br />
N<br />
Me<br />
O<br />
i. t BuOH, H 2SO 4<br />
75 ºC, 97%<br />
ii. n BuLi, THF,<br />
–40 ºC, NaBr<br />
Cl<br />
Cl<br />
clar<strong>in</strong>ex<br />
47% overall yield<br />
N<br />
Cl<br />
Cl<br />
N<br />
i. HF, BF 3, 92%<br />
ii. TEA, Tol.,<br />
N<br />
H<br />
80 ºC, 73%<br />
Me O<br />
COCl<br />
Cl<br />
N<br />
O<br />
NH t Bu<br />
N<br />
H<br />
KOH, H 2O<br />
N<br />
EtOH, D, 91%<br />
11/3/04<br />
Group Meet<strong>in</strong>g<br />
Cl<br />
N<br />
Cl<br />
i. POCl3, 89%<br />
ii. BrMg NMe<br />
THF, 50 ºC;<br />
HCl, RT, 89%<br />
O O Me<br />
Cl<br />
clarit<strong>in</strong><br />
52% overall yield
Richter<br />
SB-273005<br />
<strong>Masterpieces</strong> <strong>in</strong> <strong>Process</strong> <strong>Chemistry</strong><br />
1. Vitronect<strong>in</strong> receptor antagonist.<br />
2. Synthesis: GlaxoSmithKl<strong>in</strong>e, OPRD. 2004, 8, 738.<br />
HO<br />
MeHN N O<br />
N<br />
O<br />
DCA, [RuCl 2(R-BINAP)] 2,<br />
HO<br />
TEA, H 2, 60 ºC,<br />
MeOH, H 2O, 84%<br />
MeO 2C<br />
MeHN<br />
H<br />
O<br />
CO 2Me<br />
N OH<br />
i. PPh 3, DIAD, TBME<br />
ii. LiOH, H 2O, THF,<br />
50 ºC, 66%<br />
i. Br 2, DCM, 65%<br />
ii. itaconic acid, TEA,<br />
H<br />
Pd(OAc) 2, (o-tolyl) 3P,<br />
Bu 4NBr, MeCN, 80%<br />
HO<br />
HO 2C<br />
i. ZnCl 2, MeCN, D,<br />
ClH•H 2N CF 3<br />
OMe<br />
H<br />
ii. NaBH(OAc) 3, DMA<br />
iii. TFA, Tol., D, 72%<br />
CF 3<br />
O<br />
OMe<br />
CO 2H<br />
CO 2H<br />
HO<br />
HO 2C<br />
H 2SO 4,<br />
O<br />
MeOH, D,<br />
86%<br />
HO N<br />
MeHN N O<br />
N<br />
SB-273005<br />
18% overall yield<br />
Last Reaction = 50 kg<br />
H<br />
H<br />
CO 2H<br />
CF 3<br />
O<br />
CF 3<br />
O<br />
CO 2Me<br />
CO 2H<br />
11/3/04<br />
Group Meet<strong>in</strong>g