Bioorganic & Medicinal Chemistry Letters
Bioorganic & Medicinal Chemistry Letters
Bioorganic & Medicinal Chemistry Letters
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
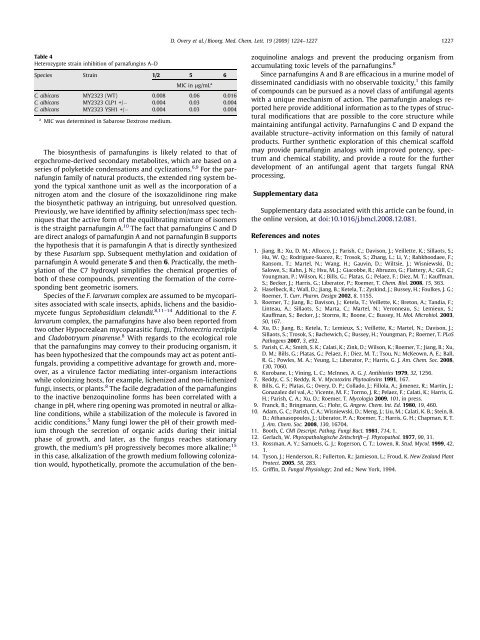
Table 4<br />
Heterozygote strain inhibition of parnafungins A–D<br />
Species Strain 1/2 5 6<br />
MIC in lg/mL a<br />
C. albicans MY2323 (WT) 0.008 0.06 0.016<br />
C. albicans MY2323 CLP1 +/ 0.004 0.03 0.004<br />
C. albicans MY2323 YSH1 +/ 0.004 0.03 0.004<br />
a MIC was determined in Sabarose Dextrose medium.<br />
The biosynthesis of parnafungins is likely related to that of<br />
ergochrome-derived secondary metabolites, which are based on a<br />
series of polyketide condensations and cyclizations. 6,9 For the parnafungin<br />
family of natural products, the extended ring system beyond<br />
the typical xanthone unit as well as the incorporation of a<br />
nitrogen atom and the closure of the isoxazolidinone ring make<br />
the biosynthetic pathway an intriguing, but unresolved question.<br />
Previously, we have identified by affinity selection/mass spec techniques<br />
that the active form of the equilibrating mixture of isomers<br />
is the straight parnafungin A. 10 The fact that parnafungins C and D<br />
are direct analogs of parnafungin A and not parnafungin B supports<br />
the hypothesis that it is parnafungin A that is directly synthesized<br />
by these Fusarium spp. Subsequent methylation and oxidation of<br />
parnafungin A would generate 5 and then 6. Practically, the methylation<br />
of the C7 hydroxyl simplifies the chemical properties of<br />
both of these compounds, preventing the formation of the corresponding<br />
bent geometric isomers.<br />
Species of the F. larvarum complex are assumed to be mycoparisites<br />
associated with scale insects, aphids, lichens and the basidiomycete<br />
fungus Septobasidium clelandii. 8,11–14 Additional to the F.<br />
larvarum complex, the parnafungins have also been reported from<br />
two other Hypocrealean mycoparasitic fungi, Trichonectria rectipila<br />
and Cladobotryum pinarense. 8 With regards to the ecological role<br />
that the parnafungins may convey to their producing organism, it<br />
has been hypothesized that the compounds may act as potent antifungals,<br />
providing a competitive advantage for growth and, moreover,<br />
as a virulence factor mediating inter-organism interactions<br />
while colonizing hosts, for example, lichenized and non-lichenized<br />
fungi, insects, or plants. 8 The facile degradation of the parnafungins<br />
to the inactive benzoquinoline forms has been correlated with a<br />
change in pH, where ring opening was promoted in neutral or alkaline<br />
conditions, while a stabilization of the molecule is favored in<br />
acidic conditions. 5 Many fungi lower the pH of their growth medium<br />
through the secretion of organic acids during their initial<br />
phase of growth, and later, as the fungus reaches stationary<br />
growth, the medium’s pH progressively becomes more alkaline; 15<br />
in this case, alkalization of the growth medium following colonization<br />
would, hypothetically, promote the accumulation of the ben-<br />
D. Overy et al. / Bioorg. Med. Chem. Lett. 19 (2009) 1224–1227 1227<br />
zoquinoline analogs and prevent the producing organism from<br />
accumulating toxic levels of the parnafungins. 8<br />
Since parnafungins A and B are efficacious in a murine model of<br />
disseminated candidiasis with no observable toxicity, 1 this family<br />
of compounds can be pursued as a novel class of antifungal agents<br />
with a unique mechanism of action. The parnafungin analogs reported<br />
here provide additional information as to the types of structural<br />
modifications that are possible to the core structure while<br />
maintaining antifungal activity. Parnafungins C and D expand the<br />
available structure–activity information on this family of natural<br />
products. Further synthetic exploration of this chemical scaffold<br />
may provide parnafungin analogs with improved potency, spectrum<br />
and chemical stability, and provide a route for the further<br />
development of an antifungal agent that targets fungal RNA<br />
processing.<br />
Supplementary data<br />
Supplementary data associated with this article can be found, in<br />
the online version, at doi:10.1016/j.bmcl.2008.12.081.<br />
References and notes<br />
1. Jiang, B.; Xu, D. M.; Allocco, J.; Parish, C.; Davison, J.; Veillette, K.; Sillaots, S.;<br />
Hu, W. Q.; Rodriguez-Suarez, R.; Trosok, S.; Zhang, L.; Li, Y.; Rahkhoodaee, F.;<br />
Ransom, T.; Martel, N.; Wang, H.; Gauvin, D.; Wiltsie, J.; Wisniewski, D.;<br />
Salowe, S.; Kahn, J. N.; Hsu, M. J.; Giacobbe, R.; Abruzzo, G.; Flattery, A.; Gill, C.;<br />
Youngman, P.; Wilson, K.; Bills, G.; Platas, G.; Pelaez, F.; Diez, M. T.; Kauffman,<br />
S.; Becker, J.; Harris, G.; Liberator, P.; Roemer, T. Chem. Biol. 2008, 15, 363.<br />
2. Haselbeck, R.; Wall, D.; Jiang, B.; Ketela, T.; Zyskind, J.; Bussey, H.; Foulkes, J. G.;<br />
Roemer, T. Curr. Pharm. Design 2002, 8, 1155.<br />
3. Roemer, T.; Jiang, B.; Davison, J.; Ketela, T.; Veillette, K.; Breton, A.; Tandia, F.;<br />
Linteau, A.; Sillaots, S.; Marta, C.; Martel, N.; Veronneau, S.; Lemieux, S.;<br />
Kauffman, S.; Becker, J.; Storms, R.; Boone, C.; Bussey, H. Mol. Microbiol. 2003,<br />
50, 167.<br />
4. Xu, D.; Jiang, B.; Ketela, T.; Lemieux, S.; Veillette, K.; Martel, N.; Davison, J.;<br />
Sillaots, S.; Trosok, S.; Bachewich, C.; Bussey, H.; Youngman, P.; Roemer, T. PLoS<br />
Pathogens 2007, 3, e92.<br />
5. Parish, C. A.; Smith, S. K.; Calati, K.; Zink, D.; Wilson, K.; Roemer, T.; Jiang, B.; Xu,<br />
D. M.; Bills, G.; Platas, G.; Pelaez, F.; Diez, M. T.; Tsou, N.; McKeown, A. E.; Ball,<br />
R. G.; Powles, M. A.; Yeung, L.; Liberator, P.; Harris, G. J. Am. Chem. Soc. 2008,<br />
130, 7060.<br />
6. Kurobane, I.; Vining, L. C.; McInnes, A. G. J. Antibiotics 1979, 32, 1256.<br />
7. Reddy, C. S.; Reddy, R. V. Mycotoxins Phytoalexins 1991, 167.<br />
8. Bills, G. F.; Platas, G.; Overy, D. P.; Collado, J.; Fillola, A.; Jimenez, R.; Martin, J.;<br />
Gonazalez del val, A.; Vicente, M. F.; Tormo, J. R.; Pelaez, F.; Calati, K.; Harris, G.<br />
H.; Parish, C. A.; Xu, D.; Roemer, T. Mycologia 2009, 101, in press.<br />
9. Franck, B.; Bringmann, G.; Flohr, G. Angew. Chem. Int. Ed. 1980, 19, 460.<br />
10. Adam, G. C.; Parish, C. A.; Wisniewski, D.; Meng, J.; Liu, M.; Calati, K. B.; Stein, B.<br />
D.; Athanasopoulos, J.; Liberator, P. A.; Roemer, T.; Harris, G. H.; Chapman, K. T.<br />
J. Am. Chem. Soc. 2008, 130, 16704.<br />
11. Booth, C. CMI Descript. Pathog. Fungi Bact. 1981, 714, 1.<br />
12. Gerlach, W. Phytopathologische Zeitschrift—J. Phytopathol. 1977, 90, 31.<br />
13. Rossman, A. Y.; Samuels, G. J.; Rogerson, C. T.; Lowen, R. Stud. Mycol. 1999, 42,<br />
1.<br />
14. Tyson, J.; Henderson, R.; Fullerton, R.; Jamieson, L.; Froud, K. New Zealand Plant<br />
Protect. 2005, 58, 283.<br />
15. Griffin, D. Fungal Physiology; 2nd ed.; New York, 1994.