Carboxymethyl chitosan and its applications - Advanced Materials ...
Carboxymethyl chitosan and its applications - Advanced Materials ...
Carboxymethyl chitosan and its applications - Advanced Materials ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
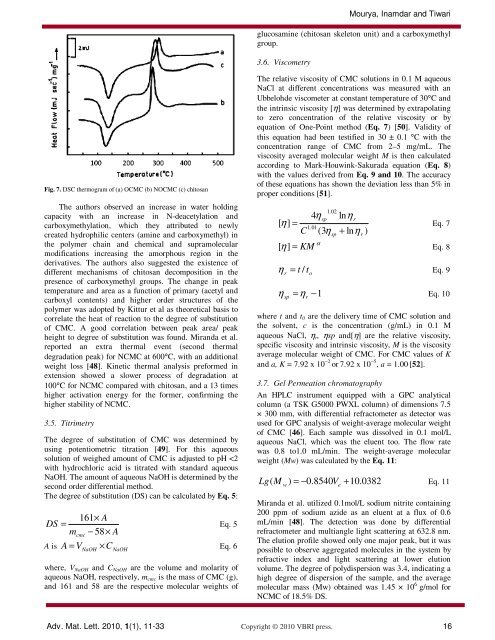
Fig. 7. DSC thermogram of (a) OCMC (b) NOCMC (c) <strong>chitosan</strong><br />
The authors observed an increase in water holding<br />
capacity with an increase in N-deacetylation <strong>and</strong><br />
carboxymethylation, which they attributed to newly<br />
created hydrophilic centers (amine <strong>and</strong> carboxymethyl) in<br />
the polymer chain <strong>and</strong> chemical <strong>and</strong> supramolecular<br />
modifications increasing the amorphous region in the<br />
derivatives. The authors also suggested the existence of<br />
different mechanisms of <strong>chitosan</strong> decomposition in the<br />
presence of carboxymethyl groups. The change in peak<br />
temperature <strong>and</strong> area as a function of primary (acetyl <strong>and</strong><br />
carboxyl contents) <strong>and</strong> higher order structures of the<br />
polymer was adopted by Kittur et al as theoretical basis to<br />
correlate the heat of reaction to the degree of substitution<br />
of CMC. A good correlation between peak area/ peak<br />
height to degree of substitution was found. Mir<strong>and</strong>a et al.<br />
reported an extra thermal event (second thermal<br />
degradation peak) for NCMC at 600°C, with an additional<br />
weight loss [48]. Kinetic thermal analysis preformed in<br />
extension showed a slower process of degradation at<br />
100°C for NCMC compared with <strong>chitosan</strong>, <strong>and</strong> a 13 times<br />
higher activation energy for the former, confirming the<br />
higher stability of NCMC.<br />
3.5. Titrimetry<br />
The degree of substitution of CMC was determined by<br />
using potentiometric titration [49]. For this aqueous<br />
solution of weighed amount of CMC is adjusted to pH