Graphical contents list
Graphical contents list
Graphical contents list
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
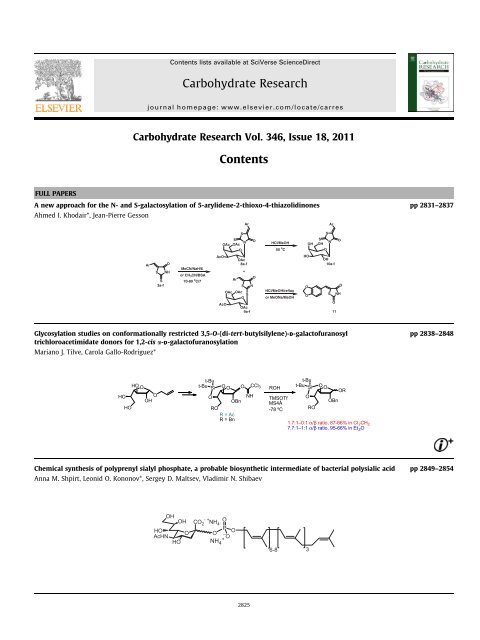
Carbohydrate Research Vol. 346, Issue 18, 2011<br />
Contents<br />
FULL PAPERS<br />
A new approach for the N- and S-galactosylation of 5-arylidene-2-thioxo-4-thiazolidinones pp 2831–2837<br />
Ahmed I. Khodair*, Jean-Pierre Gesson<br />
Ar<br />
O<br />
S NH<br />
S<br />
3a-f<br />
MeCN/NaH/6<br />
or CH 3CN/BSA<br />
70-80 o C/7<br />
AcO<br />
S<br />
N<br />
OAc OAc<br />
O<br />
AcO<br />
Ar<br />
S<br />
Ar<br />
OAc<br />
8a-f<br />
+<br />
O<br />
S N<br />
OAc OAc<br />
S<br />
O<br />
OAc<br />
9a-f<br />
O<br />
HCl/MeOH<br />
50 o C<br />
HCl/MeOH/reflux<br />
or MeONa/MeOH<br />
HO<br />
O<br />
O<br />
Ar<br />
S<br />
S<br />
N<br />
OH OH<br />
O<br />
Glycosylation studies on conformationally restricted 3,5-O-(di-tert-butylsilylene)-D-galactofuranosyl<br />
trichloroacetimidate donors for 1,2-cis a-D-galactofuranosylation<br />
Mariano J. Tilve, Carola Gallo-Rodriguez*<br />
HO<br />
HO<br />
HO O<br />
O<br />
OH<br />
Contents <strong>list</strong>s available at SciVerse ScienceDirect<br />
Carbohydrate Research<br />
journal homepage: www.elsevier.com/locate/carres<br />
t-Bu<br />
t-Bu Si<br />
O O<br />
O<br />
OBn<br />
RO<br />
R=Ac<br />
R=Bn<br />
O CCl 3<br />
NH<br />
ROH<br />
TMSOTf<br />
MS4Å<br />
-78 ºC<br />
t-Bu<br />
t-Bu Si<br />
O<br />
RO<br />
OH<br />
10a-f<br />
O O<br />
O<br />
O<br />
S NH<br />
O<br />
11<br />
OBn<br />
OR<br />
1.7:1—0:1 α/β ratio, 87-66% in Cl 2CH 2<br />
7.7:1—1:1 α/β ratio, 95-66% in Et 2O<br />
pp 2838–2848<br />
Chemical synthesis of polyprenyl sialyl phosphate, a probable biosynthetic intermediate of bacterial polysialic acid pp 2849–2854<br />
Anna M. Shpirt, Leonid O. Kononov*, Sergey D. Maltsev, Vladimir N. Shibaev<br />
OH<br />
-+<br />
OH CO2 NH4<br />
HO<br />
O O<br />
AcHN<br />
HO<br />
P<br />
O<br />
O<br />
–<br />
O<br />
NH +<br />
4<br />
2825<br />
6-8 3
2826 Contents / Carbohydrate Research 346 (2011) 2825–2830<br />
Chiral pool synthesis of calystegine A3 from 2-deoxyglucose via a Brown allylation pp 2855–2861<br />
Tina Secher Rasmussen, Henrik Helligsø Jensen*<br />
HO HO<br />
OH<br />
O<br />
OH<br />
PMBO<br />
BnO<br />
O<br />
Brown allylation<br />
PMBO<br />
BnO<br />
dr 11:1<br />
OH<br />
HO<br />
HO NH<br />
OH<br />
Calystegine A3 Preparation of 2-amino-2-C-glycosyl-acetonitriles from C-glycosyl aldehydes by Strecker reaction pp 2862–2871<br />
Szabolcs Sipos, István Jablonkai*, Orsolya Egyed, Mátyás Czugler<br />
(OPg) 4<br />
O<br />
H<br />
O<br />
H<br />
(OBn) 3<br />
(OBn) 3<br />
O<br />
CHO<br />
CHO<br />
CHO<br />
(OPg)n<br />
n = 3 or 4<br />
O<br />
Pg = Bn or Me<br />
Controversial behavior of aminoguanidine in the presence of either reducing sugars or soluble glycated bovine<br />
serum albumin<br />
Andreea Iren Serban*, Marieta Costache, Anca Dinischiotu<br />
NC<br />
H<br />
R and S<br />
N<br />
H<br />
R<br />
pp 2872–2880<br />
New triterpenoid saponins from Patrinia scabiosaefolia pp 2881–2885<br />
Liang Gao, Lin Zhang, Nan Li, Jiang-Yun Liu*, Pei-lie Cai, Shi-lin Yang<br />
HO<br />
HO<br />
HO CH 3<br />
RO<br />
1 R = Xyl<br />
2 R = H<br />
O<br />
O<br />
OH<br />
O<br />
O<br />
HOCH2 OH<br />
O<br />
2 1<br />
3 5<br />
4 6<br />
O<br />
7<br />
30<br />
29<br />
20<br />
19<br />
12<br />
11<br />
13 18<br />
25 26<br />
14<br />
9<br />
10 8 15<br />
27<br />
21<br />
28<br />
22<br />
17<br />
16<br />
24<br />
23<br />
HO<br />
HO<br />
3<br />
HO<br />
HO HO<br />
O<br />
OH<br />
O<br />
O<br />
O<br />
HO HO<br />
4<br />
HOCH 2<br />
OH O<br />
OH<br />
O<br />
OH<br />
O<br />
O<br />
HO<br />
HO HO<br />
HO CH 3<br />
O<br />
OH<br />
O<br />
O<br />
O<br />
O OH<br />
O<br />
HO HO<br />
OH<br />
O<br />
OH<br />
O<br />
O
Carbohydrate-conjugate heterobimetallic complexes: synthesis, DNA binding studies, artificial nuclease activity<br />
and in vitro cytotoxicity<br />
Sartaj Tabassum*, Rais Ahmad Khan, Farukh Arjmand, Mubashira Aziz, Aarti S. Juvekar, Surekha M. Zingde<br />
Supercoiled Form<br />
Circular Form<br />
Linear Form<br />
1 2 3 4 5 6<br />
DNA Photocleavage Pattern<br />
Form II<br />
Form III<br />
Form I<br />
pp 2886–2895<br />
Green synthesis of xylan hemicellulose esters pp 2896–2904<br />
Fatima-Zohra Belmokaddem, Catherine Pinel*, Patrick Huber, Michel Petit-Conil, Denilson Da Silva Perez<br />
O-Acetyl location on Cepacian, the principal exopolysaccharide of Burkholderia cepacia complex bacteria pp 2905–2912<br />
Paola Cescutti*, Giuseppe Impallomeni, Domenico Garozzo, Roberto Rizzo<br />
β-D-Galp-(1→2)-α-D-Rhap<br />
4<br />
OAc<br />
Contents / Carbohydrate Research 346 (2011) 2825–2830 2827<br />
OAc<br />
6<br />
3<br />
OAc<br />
13%<br />
2<br />
OAc<br />
1<br />
↓<br />
4<br />
OAc<br />
[3)-α-D-GlcpA-(1→3)-α-D-Manp-(1→3)-β-D-Glcp-(1→]n<br />
2<br />
6<br />
↑ ↑<br />
1<br />
1<br />
α-D-Galp β-D-Galp<br />
4<br />
OAc<br />
3<br />
OAc<br />
14%<br />
6<br />
OAc<br />
4 3<br />
OAc OAc<br />
Structures of building blocks in clusters of sweetpotato amylopectin pp 2913–2925<br />
Fan Zhu*, Harold Corke, Per Åman, Eric Bertoft<br />
Possible influence of building block size on the amorphous region in starch granule: Black cylinders symbolize the double-helix in the crystalline<br />
lamellae. Gray circles denote building blocks. A: Two chains from one building block with internal chain length of three interact to form a double<br />
helix with little twisted amorphous region. B: Two building blocks are close in space, but separated by a longer chain (bold line), which contributes to<br />
the formation of more twisted amorphous region.<br />
2<br />
OAc<br />
OAc
2828 Contents / Carbohydrate Research 346 (2011) 2825–2830<br />
Structural studies of the exopolysaccharide consisting of a nonasaccharide repeating unit isolated from<br />
Lactobacillus rhamnosus KL37B<br />
Sabina Górska-Frazczek*, Corine Sandström, Lennart Kenne, Jacek Rybka, Magdalena Strus, Piotr Heczko, Andrzej Gamian<br />
pp 2926–2932<br />
During the structural investigation of the exopolysaccharide it was found that the repeating unit is a nonasaccharide, which is the largest repeating unit found in LAB<br />
exopolysaccharides so far. Chemical structure of the EPS isolated from Lactobacillus rhamnosus KL37B.<br />
Computational study of mutarotation in erythrose and threose pp 2933–2939<br />
Ibon Alkorta*, Paul L.A. Popelier<br />
Open Chain<br />
H 2O<br />
(H 2O) 2<br />
Molecular dynamics simulation of the dissolution process of a cellulose triacetate-II nano-sized crystal in DMSO pp 2940–2947<br />
Daichi Hayakawa, Kazuyoshi Ueda*, Chihiro Yamane, Hitomi Miyamoto, Fumitaka Horii<br />
Furanose<br />
A reevaluation of the epimeric and anomeric relationship of glucosides and galactosides in thermotropic liquid<br />
crystal self-assemblies<br />
Rauzah Hashim*, Seyed M. Mirzadeh, Thorsten Heidelberg, Hiroyuki Minamikawa, Tanaka Yoshiaki, Akhiko Sugimura<br />
pp 2948–2956
Contents / Carbohydrate Research 346 (2011) 2825–2830 2829<br />
NOTES<br />
Stereoselective synthesis of b-N-glycosides through 2-deoxy-2-nitroglycal pp 2957–2959<br />
Shaohua Xiang, Jimei Ma, Bala Kishan Gorityala, Xue-Wei Liu*<br />
Covalent linkage of N-methyl-6-oxyquinolinium betaine to trehalose pp 2960–2964<br />
Falko Berndt, Mohsen Sajadi, Nikolaus P. Ernsting, Rainer Mahrwald*<br />
Photochemical conversion of the o-nitrobenzyl-C-glucoside to a sugar lactone pp 2965–2969<br />
Michinari Kohri*, Takuma Kimura, Yoshihiro Shinoda, Tatsuo Taniguchi, Takayuki Nakahira<br />
Green microwave-assisted synthesis of cellulose/calcium silicate nanocomposites in ionic liquids and recycled ionic<br />
liquids<br />
Ning Jia, Shu-Ming Li, Ming-Guo Ma*, Run-Cang Sun, Lei Zhu<br />
pp 2970–2974
2830 Contents / Carbohydrate Research 346 (2011) 2825–2830<br />
Investigation of a sulfate transfer during autohydrolysis of a fucoidan from the brown alga Fucus evanescens<br />
by tandem ESIMS<br />
Stanislav D. Anastyuk*, Natalia M. Shevchenko, Pavel S. Dmitrenok, Tatyana N. Zvyagintseva<br />
1<br />
H3C O<br />
4<br />
OR<br />
RO<br />
O<br />
Autohydrolysis<br />
- R=SO3 ,Ac<br />
H<br />
1<br />
3C O<br />
OR<br />
ESIMS analysis<br />
O 3<br />
OR<br />
H<br />
1<br />
3C O<br />
4<br />
OR<br />
RO<br />
O<br />
Autohydrolysis<br />
+D-Rib, D-Glc<br />
1<br />
H3C O<br />
ESIMS analysis<br />
OR<br />
O 3<br />
OR<br />
-<br />
[Fuc(SO3Na)-Na] 2-<br />
[Fuc2(SO3Na)2-2Na] 2-<br />
[FucGal(SO3Na)2-2Na] [Fuc4(SO3Na)3-3Na]<br />
4-<br />
[Fuc4(SO3Na)4-4Na] 3-<br />
[Fuc6(SO3Na)4-4Na] 4-<br />
[Fuc3(SO3Na)3-2Na] 2-<br />
[Fuc6(SO3Na)3-3Na] 3-<br />
[Fuc4(SO3Na)2-2Na] 2-<br />
-<br />
[Fuc2(SO3Na)-Na] [RibSO3Na-Na] -<br />
[GlcSO 3Na-Na] -<br />
pp 2975–2977<br />
Chemical structure of the O-polysaccharide isolated from Pectobacterium atrosepticum SCRI 1039 pp 2978–2981<br />
Małgorzata Czerwicka, Kinga Marszewska, Anna Bychowska, Halina Dziadziuszko, Krzysztof Brzozowski, Ewa Łojkowska,<br />
Piotr Stepnowski, Zbigniew Kaczyński*<br />
*Corresponding author<br />
Supplementary data available via ScienceDirect<br />
COVER<br />
Three dimensional structure of the molecular complex formed upon interaction of hevein, a plant lectin isolated from Hevea Brasiliensis tree,<br />
with the Manbeta1-4GlcNAcbeta14GlcNAcbetaAsn fragment of mammalian glycoproteins. The structure has been deduced by combination of<br />
NMR methods and molecular modelling protocols. The molecular recognition mode involves the typical interactions present in the recognition<br />
of neutral sugars by lectins, mainly CH-pi stacking interactions, hydrogen bonds and van der Waals forces. The figure has been designed by Dr.<br />
Ana Ardá and represents the first part of an ongoing collaboration with Prof. Carlo Unverzagt, at the University of Bayreuth, performed with the<br />
support of EUROCarbDB: http://www.eurocarbdb.org) and EU-NMR (http://www.eu-nmr.eu), and was presented at Tokyo during the XXV<br />
International Carbohydrate Symposium on the occasion of the Whistler Award to Jesús Jiménez-Barbero.<br />
Available online at www.sciencedirect.com<br />
Abstracted/Indexed in: Chem. Abstr.: Curr. Contents: Phys., Chem. & Earth Sci. Life Sci. Current Awareness in Bio. Sci. (CABS).<br />
Science Citation Index. Full texts are incorporated in CJELSEVIER, a file in the Chemical Journals Online database which<br />
is availabe on STN â International. Also covered in the abstract and citation database SciVerse Scopus â . Full text available on<br />
SciVerse ScienceDirect â<br />
ISSN 0008-6215